Exam 4: Reactions in Aqueous Solution
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
In which reaction does the oxidation number of hydrogen change?
Free
(Multiple Choice)
4.8/5  (20)
(20)
Correct Answer:
B
Which of the following are weak electrolytes?
HNO3
HF
NH3
LiBr
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
D
What is the concentration (M)of 39.88 mL of an unknown NaOH solution if it required 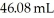 of
of 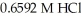 to neutralize?
to neutralize?
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
A
Mixing 1.00 mL of an aqueous solution with 9.00 mL of water represents a ________.
(Multiple Choice)
4.9/5  (25)
(25)
The net ionic equation for formation of an aqueous solution of NiI2 accompanied by evolution of CO2 gas via mixing solid NiCO3 and aqueous hydriodic acid is ________.
(Multiple Choice)
4.7/5  (30)
(30)
What volume (mL)of 7.48 × 10-2 M perchloric acid can be neutralized with 115 mL of 0.244 M sodium hydroxide?
(Multiple Choice)
4.7/5  (27)
(27)
A titration reached the equivalence point when 16.1 mL of 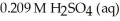 was added to
was added to 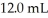 of NaOH (aq)of unknown concentration.What is the concentration (M)of this unknown NaOH solution?
of NaOH (aq)of unknown concentration.What is the concentration (M)of this unknown NaOH solution?
(Multiple Choice)
4.9/5  (26)
(26)
How many moles of CaCl2 are formed in the neutralization of 138 mL of 0.352 M Ca(OH)2 with aqueous HCl?
(Short Answer)
4.9/5  (32)
(32)
How many moles of Na+ are present in 343 mL of a 1.27 M solution of Na2SO4?
(Multiple Choice)
4.8/5  (40)
(40)
How many grams of H3PO4 are in 145 mL of a 3.50 M solution of H3PO4?
(Multiple Choice)
4.8/5  (45)
(45)
What are the spectator ions in the reaction between KOH (aq)and HNO3 (aq)?
(Multiple Choice)
4.9/5  (29)
(29)
How many moles of Cu2+ ions are present in 0.100 L of a 0.100 M solution of CuCl2?
(Short Answer)
4.8/5  (37)
(37)
How many grams of H3PO4 are in 175 mL of a 4.00 M solution of H3PO4?
(Multiple Choice)
4.7/5  (35)
(35)
What is the molarity of iodide ion in a solution prepared by mixing 20.0 mL of 0.100 M HI and 5.00 mL of 0.200 M KI?
(Multiple Choice)
4.7/5  (41)
(41)
Of the following elements,________ is the most easily oxidized. lithium
Zinc
Hydrogen
Aluminum
Gold
(Multiple Choice)
4.7/5  (34)
(34)
Combining aqueous solutions of BaI2 and Na2SO4 affords a precipitate of BaSO4.Which ions are spectator ions in the reaction?
(Multiple Choice)
4.9/5  (28)
(28)
Which species is oxidized in the reaction below?
Au(s)+ 3NO3-(aq)+ 6H+(aq)→ Au3+(aq)+ NO(g)+ 3H2O (l)
H+
N+5
O2-
H2O
Au
(Multiple Choice)
4.8/5  (25)
(25)
A 17.5 mL sample of an acetic acid (CH3CO2H)solution required 29.6 mL of 0.250 M NaOH for neutralization.The concentration of acetic acid was ________ M.
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)