Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The product of the nuclear reaction in which 40Ar is subjected to neutron capture followed by alpha emission is ________.
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
A
The half-life of a radionuclide ________.
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
A
The amount of fissionable material necessary to maintain a chain reactions is called the ________.
Free
(Short Answer)
4.8/5  (40)
(40)
Correct Answer:
critical mass
The three radioactive series that occur in nature end with what element?
(Multiple Choice)
4.8/5  (40)
(40)
What is the age in years of a mineral sample that has a mass ratio of 40Ar to 40K of 0.330? Potassium-40 decays to argon-40 with a half-life of 1.27 × 109 yr.
(Multiple Choice)
4.9/5  (28)
(28)
Carbon-11,fluorine-18,oxygen-15 and nitrogen-13 are all used in the clinical diagnostic technique known as ________.
(Short Answer)
4.8/5  (34)
(34)
Carbon-11 decays by positron emission.The decay occurs with a release of 2.87 × 1011 J per mole of carbon-11.What mass (g)is converted to energy when 5.00 g of 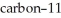 undergoes this radioactive decay?
undergoes this radioactive decay?
(Multiple Choice)
4.9/5  (40)
(40)
A ________ is a highly reactive substance that contains one or more unpaired electrons.
(Multiple Choice)
4.8/5  (35)
(35)
The formation of krypton from rubidium decay is a result of ________.
(Multiple Choice)
4.9/5  (32)
(32)
Of the following processes,which one changes the atomic number?
(Multiple Choice)
4.9/5  (33)
(33)
In terms of binding energy per nucleon,what element divides fission and fusion processes?
(Multiple Choice)
4.8/5  (38)
(38)
What is the rate constant for the decay of some unknown radioactive compound if the half-life for the beta decay is 1.3 × 109 years?
(Short Answer)
4.8/5  (40)
(40)
The conversion of matter to energy and mass loss occurs in ________ reactions?
(Short Answer)
4.8/5  (38)
(38)
The relative biological effectiveness (RBE)is tenfold greater for gamma radiation than for alpha radiation.
(True/False)
4.8/5  (35)
(35)
What is the rate constant (in min-1)for the decay of this radionuclide?
(Multiple Choice)
4.8/5  (33)
(33)
The mass of a proton is 1.673 × 10-24 g.The mass of a neutron is 1.675 × 10-24 g.The mass of the nucleus of an 56Fe atom is 9.289 × 10-23 g.What is the nuclear binding energy (in J)for a 56Fe nucleus? 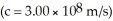
(Multiple Choice)
4.8/5  (23)
(23)
Showing 1 - 20 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)