Exam 1: Introduction: Matter, energy, and Measurement
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
A difference in one degree of temperature is the smallest on the ________ temperature scale.
Free
(Multiple Choice)
4.8/5  (48)
(48)
Correct Answer:
C
An inert atmosphere was obtained by adding nitrogen in a room that measures 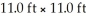 and has a 8.00 ft ceiling.How many liters of nitrogen were used to fill the room?
and has a 8.00 ft ceiling.How many liters of nitrogen were used to fill the room? 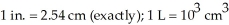
Free
(Multiple Choice)
4.9/5  (27)
(27)
Correct Answer:
A
The density of silver is 10.5 g/cm3.A piece of silver with a mass of 61.3 g would occupy a volume of ________ cm3.
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
E
Gold has a density of 0.01932 kg/cm3.What is the mass (in kg)of a 92.5 cm3 sample of gold?
(Multiple Choice)
4.7/5  (29)
(29)
The density of a gold nugget is 19.3 g/cm3.If the volume of the gold nugget is 0.00369 L,the mass of the nugget is ________ g.
(Multiple Choice)
4.7/5  (33)
(33)
The volume of a regular cylinder is V =πr2h.What is the volume  of a cylinder of radius 2.34 cm and height 19.91 cm expressed to the correct number of significant figures?
of a cylinder of radius 2.34 cm and height 19.91 cm expressed to the correct number of significant figures?
(Multiple Choice)
4.8/5  (28)
(28)
Round the following number to four significant figures and express the result in standard exponential notation: 0.00222755
(Multiple Choice)
4.9/5  (32)
(32)
An iron mine produces 2.18 × 105 US tons of raw ore on a daily basis which contains 21.47% elemental iron.How many pounds of elemental iron would the mine produce over a span of one year? (Assume the mine operates 365 days per year.)(1 US ton = 2000 lbs.)
(Multiple Choice)
4.8/5  (42)
(42)
An object measuring 0.76 decimeters will have a length of ________ centimeters.
(Multiple Choice)
4.7/5  (39)
(39)
Iron has a density of 7.9 g/cm3.What is the mass of a cube of iron with the length of one side equal to 55.0 mm?
(Multiple Choice)
4.8/5  (39)
(39)
If an object is accelerating at a rate of 25 m/s2,how long (in seconds)will it take to reach a speed of 550 m/s? (Assume an initial velocity of zero.)
(Multiple Choice)
4.9/5  (42)
(42)
A small amount of salt dissolved in water is an example of a ________.
(Multiple Choice)
5.0/5  (28)
(28)
Showing 1 - 20 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)