Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
When a hydronium ion concentration equals 1 × 10-10 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.7/5  (39)
(39)
Why do we use the pH scale to indicate the acidity of a solution rather than simply stating the concentration of hydronium ions?
(Multiple Choice)
4.8/5  (39)
(39)
In one type of fuel cell the following oxidation-reduction reactions are taking place: 2 H2 + O2 → 2 H2O
What is the fuel?
(Multiple Choice)
4.9/5  (33)
(33)
The oxidation of iron to rust is a problem structural engineers need to be concerned about, but the oxidation of aluminum to aluminum oxide is not so much of a problem. Why?
(Multiple Choice)
4.8/5  (34)
(34)
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-5M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.7/5  (43)
(43)
What would be the best explanation for the fact that most natural water has a pH of about 5.6?
(Multiple Choice)
4.8/5  (48)
(48)
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-11M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.9/5  (31)
(31)
What might the relationship be between an element's electronegativity and its ability to behave as an oxidizing agent?
(Multiple Choice)
4.8/5  (33)
(33)
According to the following reaction, which molecule is acting as a base? H2O + H2SO4 → H3O+ + HSO4-
(Multiple Choice)
4.8/5  (30)
(30)
Jewelry is often manufactured by electroplating an expensive metal such as gold over a cheaper metal. A setup for this process can be sketched as follows: 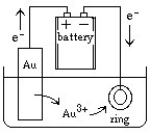 What would happen if the battery connections were suddenly reversed?
What would happen if the battery connections were suddenly reversed?
(Multiple Choice)
4.9/5  (30)
(30)
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
(Multiple Choice)
4.8/5  (29)
(29)
According to the following reaction, which molecule is acting as a base? H2O + NH3 → OH- + NH4+
(Multiple Choice)
4.8/5  (43)
(43)
Along with the pH scale, there is the pOH scale, which indicates the level of "basicity" in a solution. Accordingly, pOH = -log[OH-]. What is the sum of the pH and the pOH of a solution always equal to?
(Multiple Choice)
4.7/5  (30)
(30)
Aluminum metal undergoes the same basic corrosion process that iron does yet it does not decompose as rapidly. Why?
(Multiple Choice)
4.8/5  (37)
(37)
The following set of redox reactions takes place as shown. Container A Container B
Fe → Fe+2 + 2e- Cu +2 + 2e- → Cu
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in container A) and a piece of copper (in container B) and a salt-bridge connecting the two containers, which way do the positive ions in the salt-bridge flow?
(Multiple Choice)
4.9/5  (36)
(36)
How does connecting a metal like iron with a wire to a metal that is easier to oxidize like zinc help prevent corrosion of the iron?
(Multiple Choice)
4.9/5  (33)
(33)
As the hydronium ion concentration increases, the pH ________.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 141 - 160 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)