Exam 18: Two Classes of Chemical Reactions
Exam 1: Patterns of Motion and Equilibrium94 Questions
Exam 2: Newtons Laws of Motion109 Questions
Exam 3: Momentum and Energy128 Questions
Exam 4: Gravity, Projectiles, and Satellites114 Questions
Exam 5: Fluid Mechanics120 Questions
Exam 6: Thermal Energy and Thermodynamics100 Questions
Exam 7: Heat Transfer and Change of Phase115 Questions
Exam 8: Static and Current Electricity144 Questions
Exam 9: Magnetism and Electromagnetic Induction105 Questions
Exam 10: Waves and Sound120 Questions
Exam 11: Light146 Questions
Exam 12: Atoms and the Periodic Table128 Questions
Exam 13: The Atomic Nucleus and Radioactivity124 Questions
Exam 14: Elements of Chemistry49 Questions
Exam 15: How Atoms Bond and Molecules Attract150 Questions
Exam 16: Mixtures141 Questions
Exam 17: How Chemicals React118 Questions
Exam 18: Two Classes of Chemical Reactions182 Questions
Exam 19: Organic Compounds98 Questions
Exam 20: Rocks and Minerals170 Questions
Exam 21: Plate Tectonics and Earths Interior175 Questions
Exam 22: Shaping Earths Surface175 Questions
Exam 23: Geologic Timereading the Rock Record145 Questions
Exam 24: The Oceans, Atmosphere, and Climatic Effects172 Questions
Exam 25: Driving Forces of Weather145 Questions
Exam 26: The Solar System87 Questions
Exam 27: Stars and Galaxies84 Questions
Exam 28: The Structure of Space and Time55 Questions
Exam 29: Prologue: the Nature of Science22 Questions
Select questions type
A major source of chlorine gas, Cl2, is from the electrolysis of brine, which is concentrated salt water, NaCl (aq). Which of the following is the balanced chemical reaction for this electrolysis reaction?
(Multiple Choice)
4.9/5  (38)
(38)
Identify the acid or base behavior of each substance in these reactions: HSO4- + H2O ⇌ OH- + H2S 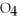
(Multiple Choice)
4.9/5  (43)
(43)
What would be the concentration of hydronium ions in a solution that had a pH = -3? Why would such a solution be impossible to prepare?
(Multiple Choice)
4.9/5  (33)
(33)
Your car lights were left on while you were shopping, and now your car battery is dead. Has the pH of the battery fluid increased or decreased?
(Multiple Choice)
4.9/5  (36)
(36)
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-1M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.9/5  (24)
(24)
In a battery, the following oxidation-reduction reactions are taking place: Mn2O3 + ZnO → 2 MnO2 + Zn
What is undergoing an oxidation as written?
(Multiple Choice)
4.8/5  (39)
(39)
What best describes what happens when an acid such as HCl is mixed with water?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following statements about strong and weak acids is not true?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the above illustrations shows a basic aqueous solution?
(Multiple Choice)
4.9/5  (42)
(42)
Chemical equations need to be balanced not only in terms of the number of atoms, but also by the charge. In other words, just as there should be the same number of atoms before and after the arrow of an equation, there should be the same charge. What set of coefficients is necessary to balance the following chemical equation? ____ Ce4+ + ____ Cl+ → ____ Ce3+ + ____ Cl2
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following statements best describes a cathode?
(Multiple Choice)
4.9/5  (31)
(31)
What is the primary difference between a fuel cell and a battery?
(Multiple Choice)
4.8/5  (38)
(38)
If you had a 1 M solution of a weak base what would be its pH?
(Multiple Choice)
4.9/5  (33)
(33)
Arrange the following images of an aqueous base solution in order of increasing base strength: 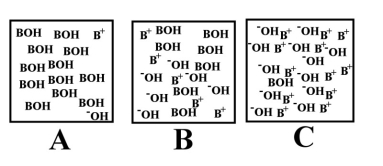
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements about electrochemistry is not true?
(Multiple Choice)
4.9/5  (31)
(31)
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-8M? [H3O+] × [OH-] = Kw = 1 × 10-14
(Multiple Choice)
4.9/5  (36)
(36)
Why don't the electrodes of a fuel cell deteriorate the way the electrodes of a battery do?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 101 - 120 of 182
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)