Exam 41: Atomic Structure
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
If the orbital quantum number is l = 4,which one of the following is a possible value for the principal quantum number n?
(Multiple Choice)
4.7/5  (41)
(41)
An electron in a hydrogen atom is in the n = 7 shell.How many possible values of the orbital quantum number l could it have?
(Multiple Choice)
4.9/5  (39)
(39)
Consider the n = 9 shell.
(a)What is the largest value of the orbital quantum number,l,in this shell?
(b)How many electrons can be placed in this shell?
(Short Answer)
4.8/5  (32)
(32)
An electron in a hydrogen atom has orbital quantum number l = 4.How many possible values of the magnetic quantum number ml could it have?
(Multiple Choice)
4.8/5  (40)
(40)
The only VALID electron state and shell designation among the following is
(Multiple Choice)
4.9/5  (40)
(40)
How many possible sets of quantum numbers (electron states)are there in the 5f subshell?
(Multiple Choice)
4.8/5  (37)
(37)
An electron in a hydrogen atom has principal quantum number n = 4.How many possible values of the orbital quantum number l could it have?
(Multiple Choice)
4.8/5  (35)
(35)
For each value of the principal quantum number n,what are the possible values of the electron spin quantum number ms? (There may be more than one correct choice.)
(Multiple Choice)
4.9/5  (37)
(37)
A three-dimensional potential well has potential U0 = 0 in the region 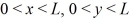 ,and
,and 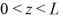 and infinite potential otherwise.The ground state energy of a particle in the well is E0.What is the energy of the first excited state,and what is the degeneracy of that state?
and infinite potential otherwise.The ground state energy of a particle in the well is E0.What is the energy of the first excited state,and what is the degeneracy of that state?
(Multiple Choice)
4.8/5  (36)
(36)
Doubly-ionized lithium is a hydrogen-like ion,in which the nucleus with charge 3e is orbited by a single electron.What wavelength light would be emitted from Li++ as the electron dropped from the n = 4 to the n = 3 orbit?
(Multiple Choice)
4.9/5  (37)
(37)
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.Which one of the following numbers could be the principal quantum number n of the electron?
(Multiple Choice)
4.7/5  (33)
(33)
A muonic hydrogen atom is a proton orbited by a muon (a particle with the same charge as an electron and 207 times its mass)in which the mass of the muon is significant relative to the mass of the proton.What would be the radius of the smallest muon orbit in a muonic hydrogen atom?
(Multiple Choice)
4.8/5  (44)
(44)
An atom with 5 electrons is in its ground state.How many electrons are in its outermost shell?
(Short Answer)
4.9/5  (42)
(42)
What is the minimum speed needed by a ground-state hydrogen atom for its kinetic energy to be enough to ionize the atom in a collision? (1 eV = 1.60 × 10-19 J, mH≈ mproton =1.67 x 10-27 kg)
(Multiple Choice)
4.7/5  (28)
(28)
If the principal quantum number of an electron is n = 5,which one of the following is NOT an allowed magnetic quantum number ml for the electron?
(Multiple Choice)
4.9/5  (33)
(33)
If two electrons in the same atom have the same four quantum numbers,then they must have the same energy.
(Multiple Choice)
4.7/5  (38)
(38)
What is the correct electronic configuration for ground state carbon,which has 6 electrons?
(Multiple Choice)
4.8/5  (34)
(34)
An atom in a state with its orbital quantum number l = 1 decays to its ground state (with l = 0).A photon of wavelength 630.000 nm is emitted in the process.When the same process takes place in the presence of an intense magnetic field,the following change in the spectrum is observed.With the magnetic field present,one of the emitted lines observed now has a wavelength of 630.030 nm.Which of the following wavelengths would you expect to be also present?
(Multiple Choice)
4.9/5  (33)
(33)
What is the radius of the smallest electron orbit in a singly-ionized helium atom?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 21 - 40 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)