Exam 42: Molecules and Condensed Matter
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
A vibrating diatomic molecule in its ground state has energy E.What is the energy of the same molecule in its second EXCITED state?
(Multiple Choice)
4.7/5  (33)
(33)
A rotating diatomic molecule has rotational quantum number l.The energy DIFFERENCE between adjacent energy levels
(Multiple Choice)
4.8/5  (36)
(36)
The moment of inertia of a fluorine ( 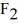 )molecule is
)molecule is 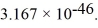 What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J • s, h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J • s, h = 1.055 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.7/5  (36)
(36)
A diatomic molecule has 2.6 × 10-5 eV of rotational energy in the l = 2 quantum state.What is its rotational energy in the l = 1 quantum state?
(Multiple Choice)
4.9/5  (35)
(35)
The Fermi level (energy)of a metal is 5.5 eV.What is the number of conduction electrons per unit volume for this metal? (mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J, 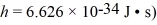
(Multiple Choice)
4.9/5  (29)
(29)
Silver has a Fermi level (energy)of 5.5 eV.At 0 K,at what speed would the electrons be moving if they had kinetic energy equal to the Fermi energy? (This speed is known as the Fermi speed.)(mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J,h = 6.626 × 10-34 J • s)
(Multiple Choice)
4.8/5  (47)
(47)
The spacing of the atoms (treated as point masses)in the H2 molecule is 7.4 × 10-11 m.What is the energy of the l = 1 rotational level? (1 eV = 1.60 × 10-19 J, 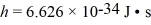 , h = 1.055 × 10-34 J ∙ s,mh ≈ mproton = 1.67 × 10-27 kg)
, h = 1.055 × 10-34 J ∙ s,mh ≈ mproton = 1.67 × 10-27 kg)
(Multiple Choice)
4.8/5  (36)
(36)
The Fermi energy of rubidium at a temperature of 5 K is 1.85 eV.An electron state in rubidium is 0.007 eV above the Fermi level.What is the probability that this state is occupied at a temperature of 9K? (mel = 9.11 × 10-31 kg,h = 6.626 × 10-34 J • s,Boltzmann constant = 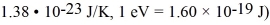
(Multiple Choice)
4.9/5  (36)
(36)
For a solid in which the occupation of the energy states is given by the Fermi-Dirac distribution,the probability that a certain state is occupied at a temperature T0 is 0.70.If the temperature is doubled to 2T0,what is the probability that the same state is occupied? Assume that the Fermi energy does not change with temperature.
(Short Answer)
4.7/5  (33)
(33)
Showing 21 - 31 of 31
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)