Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
A certain compound has a molar mass of 150 g/mol.Which is a possible empirical formula for this compound?
(Multiple Choice)
4.9/5  (31)
(31)
If the complete combustion of an unknown mass of ethylene produces 16.0 g CO2,what mass of ethylene is combusted?
C2H4(g)+ 3 O2(g) 2 CO2(g)+ 2 H2O(g)
(Multiple Choice)
4.8/5  (31)
(31)
Calculate the number of moles of O2 required to react with phosphorus to produce 4.76 g of P4O6.
(Molar mass P4O6 = 219.9 g/mol)
(Multiple Choice)
4.8/5  (44)
(44)
PCl3 can be produced from the reaction of P4 with Cl2.
P4(s)+ 6 Cl2(g) 4 PCl3(g)
If 1.00 g P4 reacts with 1.00 g Cl2,which reactant is the limiting reactant? What mass of product may be produced?
(Essay)
4.8/5  (37)
(37)
How many moles of sulfate ions are there in a 0.387-L solution of 0.927 M Al2(SO4)3?
(Multiple Choice)
4.8/5  (36)
(36)
A 1.850 g mixture of SrCO3 and SrO is heated.The SrCO3 decomposes to SrO and CO2.What was the mass percentage of SrCO3 in the mixture if the mass after heating is 1.445 g?
(Multiple Choice)
4.8/5  (33)
(33)
A 1.185 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 2.264 g CO2 and 1.390 g H2O.What mass of oxygen is contained in the original sample?
(Multiple Choice)
4.7/5  (43)
(43)
The empirical formula of ethylene is CH2.An experimental determination of the molar mass of ethylene yields the value of 28 g/mol.What is the formula of ethylene?
(Multiple Choice)
4.9/5  (36)
(36)
A mass of 0.4113 g of an unknown acid,HA,is titrated with NaOH.If the acid reacts with 28.10 mL of 0.1055 M NaOH(aq),what is the molar mass of the acid?
(Multiple Choice)
4.9/5  (38)
(38)
If 48.8 g of O2 is mixed with 48.8 g of H2 and the mixture is ignited,what is the maximum mass of water that may be produced?
(Multiple Choice)
4.9/5  (37)
(37)
Sulfur hexafluoride is produced by reacting elemental sulfur with fluorine gas.S8(s)+ 24 F2(g) 8 SF6(g)
What is the percent yield if 18.3 g SF6 is isolated from the reaction of 10.0 g S8 and 30.0 g F2?
(Multiple Choice)
4.7/5  (48)
(48)
What is the molarity of an NaI solution that contains 5.3 g of NaI in 41.0 mL of solution?
(Multiple Choice)
4.9/5  (30)
(30)
What minimum mass of copper (II)nitrate must be added to 30.0 mL of a 0.0387 M phosphate solution in order to completely precipitate all of the phosphate as solid copper (II)phosphate?
2PO43-(aq)+ 3Cu(NO3)2(aq) Cu3(PO4)2(s)+ 6NO3-(aq)
(Multiple Choice)
4.9/5  (31)
(31)
A 5.95-g sample of AgNO3 is reacted with excess BaCl2 according to the equation
2AgNO3(aq)+ BaCl2(aq) 2AgCl(s)+ Ba(NO3)2(aq)
To give 3.75 g of AgCl.What is the percent yield of AgCl?
(Multiple Choice)
4.8/5  (43)
(43)
A 3.361 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 6.295 g CO2 and 3.866 g H2O.What is the empirical formula of the hydrocarbon?
(Multiple Choice)
4.9/5  (41)
(41)
A dilute solution is prepared by transferring 45.00 mL of a 0.5616 M stock solution to a 400.0 mL volumetric flask and diluting to mark.What is the molarity of this dilute solution?
(Multiple Choice)
4.9/5  (44)
(44)
Sulfur trioxide,SO3,is made from the oxidation of SO2as follows: 2SO2 + O2 2SO3
A 21-g sample of SO2 gives 18 g of SO3.The of SO3 is .
(Multiple Choice)
4.8/5  (32)
(32)
A 4.541 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 8.674 g CO2 and 5.328 g H2O.What is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (33)
(33)
The following equation: A = 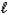 C (where
C (where 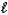 is path length and C is concentration)is known as the _____-Lambert law.
is path length and C is concentration)is known as the _____-Lambert law.
(Short Answer)
4.8/5  (37)
(37)
Showing 41 - 60 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)