Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Naphthalene,a hydrocarbon,has an approximate molar mass of 128 g/mole.If the combustion of 0.6400 g naphthalene produces 0.3599 g H2O and 2.1977 g CO2,what is the molecular formula of this compound?
(Multiple Choice)
4.7/5  (34)
(34)
The reaction of HCl with NaOH is represented by the equation
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
What volume of 0.686 M HCl is required to titrate 42.8 mL of 0.334 M NaOH?
(Multiple Choice)
4.9/5  (42)
(42)
The commercial production of phosphoric acid,H3PO4,can be represented by the equation
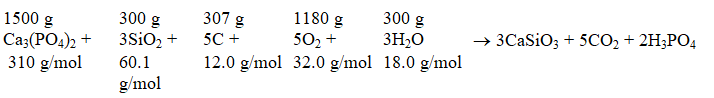 The molar mass for each reactant is shown the reactant,and the mass of each reactant for this problem is given Which substance is the limiting reactant?
The molar mass for each reactant is shown the reactant,and the mass of each reactant for this problem is given Which substance is the limiting reactant?
(Multiple Choice)
4.9/5  (34)
(34)
A 2.841 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 7.794 g CO2 and water.What mass of hydrogen is contained in the original sample?
(Multiple Choice)
4.7/5  (45)
(45)
Nitric oxide is made from the oxidation of ammonia.What mass of nitric oxide can be made from the reaction of 8.00 g NH3 with 17.0 g O2?
4 NH3(g)+ 5 O2(g) 4 NO(g)+ 6 H2O(g)
(Multiple Choice)
4.8/5  (33)
(33)
Soft drink bottles are made of polyethylene terephthalate (PET),a polymer composed of carbon,hydrogen,and oxygen.If 1.9022 g PET is burned in oxygen it produces 0.6585 g H2O and 4.0216 g CO2.What is the empirical formula of PET?
(Multiple Choice)
4.7/5  (33)
(33)
How many moles of sodium bromide can be produced from the reaction of 1.03 moles of sodium with 0.650 moles of bromine gas?
2 Na(s)+ Br2(g) 2 NaBr(s)
(Multiple Choice)
4.7/5  (36)
(36)
In combustion analysis,the gases produced by the combustion of a hydrocarbon are passed through a tube containing finely divided NaOH supported on asbestos.The purpose of the NaOH is to absorb ________ produced by the combustion reaction.
(Short Answer)
4.7/5  (27)
(27)
If 0.1800 g of impure soda ash (Na2CO3)is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash?
Na2CO3(aq)+ 2 HCl(aq) 2 NaCl(aq)+ H2O(  )+ CO2(g)
)+ CO2(g)
(Multiple Choice)
4.8/5  (50)
(50)
Chlorine was passed over 2.02 g of heated titanium,and 6.50 g of a chloride-containing compound of Ti was obtained.What is the empirical formula of the chloride-containing compound?
(Multiple Choice)
4.8/5  (45)
(45)
Polyethylene is a polymer consisting of only carbon and hydrogen.If 2.300 g of the polymer is burned in oxygen it produces 2.955 g H2O and 7.217 g CO2.What is the empirical formula of polyethylene?
(Multiple Choice)
4.8/5  (38)
(38)
The reaction of 5.07 g N2 with 0.722 g H2 produces 1.27 g NH3.The percent yield of this reaction is ________.
(Short Answer)
4.9/5  (42)
(42)
Consider the fermentation reaction of glucose:
C6H12O6 2C2H5OH + 2CO2
A 1.00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast.If 67.8 g of C2H5OH was obtained,what was the percent yield of C2H5OH?
(Multiple Choice)
4.9/5  (44)
(44)
If 15.0 g N2 and 2.00 g H2 react to produce 1.38 g NH3,what is the percent yield of the reaction?
N2(g)+ 3 H2(g) 2 NH3(g)
(Multiple Choice)
4.8/5  (31)
(31)
Showing 61 - 76 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)