Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
When strongly heated,boric acid breaks down to boric oxide and water.What mass of boric oxide is formed from the decomposition of 15.0 g B(OH)3?
2 B(OH)3(s) B2O3(s)+ 3 H2O(g)
(Multiple Choice)
4.8/5  (39)
(39)
An impure sample of benzoic acid (C6H5COOH,122.12 g/mol)is titrated with 0.8067 M NaOH.A 5.109-g sample requires 36.97 mL of titrant to reach the endpoint.What is the percent by mass of benzoic acid in the sample?
C6H5COOH(aq)+ NaOH(aq) NaC6H5COO(aq)+ H2O(l)
(Multiple Choice)
4.9/5  (38)
(38)
A 50.00-mL sample of a weak monoprotic acid is titrated with 0.0955 M NaOH.At the endpoint,it is found that 32.56 mL of titrant was used.What was the concentration of the weak acid?
(Multiple Choice)
4.9/5  (46)
(46)
The pH of an aqueous NaOH solution is 12.83.What is the hydrogen ion concentration of this solution?
(Multiple Choice)
4.9/5  (32)
(32)
Calibration of a spectrophotmeter using a series of green dye containing solutions provides a set of data which follows the Beer-Lambert Law,with a trendline of y = 1.070 103x (y-axis = absorbance,x-axis = molar concentration).What is the molar concentration of a solution with an absorbance of 0.3131?
(Multiple Choice)
4.8/5  (39)
(39)
The pH of a vinegar solution is 4.15.What is the H3O+ concentration of the solution?
(Multiple Choice)
4.8/5  (34)
(34)
A compound consists of only C and F.It contains 38.71% C by mass.What is the empirical formula of the compound?
(Multiple Choice)
4.7/5  (32)
(32)
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows:
Cu2S(s)+ O2(g) 2Cu(s)+ SO2(g)
What mass of copper(I)sulfide is required in order to prepare 0.610 kg of copper metal?
(Multiple Choice)
4.9/5  (34)
(34)
The reaction of 0.779 g K with O2 forms 1.417 g potassium superoxide,a substance used in self-contained breathing devices.Determine the formula for potassium superoxide.
(Multiple Choice)
4.9/5  (44)
(44)
Potassium hydrogen carbonate decomposes with heat as follows:
2KHCO3(s) K2CO3(s)+ CO2(g)+ H2O(l)
How many moles of potassium carbonate will be produced if 201 g of potassium hydrogen carbonate are heated?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following statements is/are CORRECT?
1)Absorbance is directly proportional to the intensity of the incident light.
2)Absorbance is inversely proportional to the analyte concentration.
3)Absorbance is directly proportional to the path length of the light.
(Multiple Choice)
4.9/5  (37)
(37)
The combustion of 0.0272 mole of a hydrocarbon produces 1.9609 g H2O and 3.5927 g CO2.What is the molar mass of the hydrocarbon?
(Multiple Choice)
4.8/5  (38)
(38)
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows:
Cu2S(s)+ O2(g) 2Cu(s)+ SO2(g)
If the reaction of 0.630 kg of copper(I)sulfide with excess oxygen produces 0.190 kg of copper metal,what is the percent yield?
(Multiple Choice)
4.7/5  (39)
(39)
An aqueous nitric acid solution has a pH of 2.15.What mass of HNO3 is present in 20.0 L of this solution?
(Multiple Choice)
4.8/5  (34)
(34)
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation:
Rh2(SO4)3(aq)+ 6NaOH(aq) → 2Rh(OH)3(s)+ 3Na2SO4(aq)
If 6.20 g of rhodium(III)sulfate reacts with excess sodium hydroxide,what mass of rhodium(III)hydroxide may be produced?
(Multiple Choice)
4.9/5  (25)
(25)
What mass of Na2CO3 is present in 0.250 L of a 0.852 M Na2CO3 solution?
(Multiple Choice)
4.8/5  (35)
(35)
How many grams of dioxygen are required to completely burn 4.3 g of C2H5OH?
(Multiple Choice)
4.8/5  (36)
(36)
Zn reacts with hydrochloric acid.
Zn(s)+ 2 HCl(aq) ZnCl2(aq)+ H2(g)
What volume of 3.05 M HCl(aq)will react with 25.0 g Zn(s)?
(Multiple Choice)
4.9/5  (34)
(34)
If 5.00 g Br2 and 1.10 g NH3 react according to the equation below,what is the maximum mass of ammonium bromide produced?
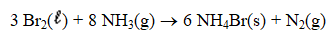
(Multiple Choice)
4.8/5  (38)
(38)
How many moles of Mg3P2(s)can be produced from the reaction of 0.14 mol Mg(s)with 0.020 mol P4(s)?
6 Mg(s)+ P4(s) 2 Mg3P2(s)
(Multiple Choice)
4.9/5  (39)
(39)
Showing 21 - 40 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)