Exam 7: The Structure of Atoms
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
A light emitting diode (L.E.D.)emits photons with an energy of 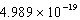 J.What is the energy per mole of photons emitted?
J.What is the energy per mole of photons emitted?
(Multiple Choice)
4.8/5  (43)
(43)
What is the value of the spin quantum number for an electron in a 4p orbital?
(Multiple Choice)
4.9/5  (40)
(40)
All of the following sets of quantum numbers are allowed EXCEPT
(Multiple Choice)
4.9/5  (46)
(46)
Which of the following is/are correct postulates of Bohr's theory of the hydrogen atom ?
1)The energy of an electron in an atom is quantized (i.e.only specific energy values are possible).
2)The principal quantum number (n),specifies each unique energy level.
3)An electron transition from a lower energy level to a higher energy level results in an emission of a photon of light.
(Multiple Choice)
4.7/5  (31)
(31)
According to Heisenberg's ________ principle,it is impossible to simultaneously measure the exact location and energy of an electron.
(Short Answer)
4.8/5  (35)
(35)
Which of the following ranks regions of the electromagnetic spectrum in proper order from highest to lowest frequency.
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following statements is/are CORRECT?
1)FM radio waves have longer wavelengths than visible radiation and AM radio waves have shorter wavelengths than visible radiation.
2)Magnetic resonance imaging (MRI)uses a mixture of x-rays and gamma rays.
3)Exposure to ultraviolet (UV)radiation can lead to sunburns.
(Multiple Choice)
4.8/5  (45)
(45)
The difference in energy between adjacent energy levels in an atom _____ as n increases.
(Short Answer)
5.0/5  (32)
(32)
The electron in a hydrogen atom,originally in level 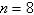 ,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? (
,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? ( 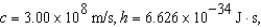
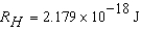 )
)
(Multiple Choice)
4.9/5  (41)
(41)
If the de Broglie wavelength of an electron is 44 nm,what is its velocity? The mass of an electron is 9.11 10-31 kg.
(Multiple Choice)
4.8/5  (38)
(38)
The size of an electron orbital is often chosen to be the distance within which 90% of the electron density is found.Why is the orbital radius not chosen to be the distance within which 100% of the electron density is found?
(Essay)
4.9/5  (40)
(40)
What is the wavelength of a photon that has an energy of 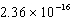 J?
J?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following statements concerning quantum mechanics is/are true?
1)The behavior of submicroscopic particles can sometimes be described as waves.
2)Quantum mechanics limits us to making statistical statements about the location of an electron in an atom.
3)The uncertainty principle is important only for particles of very small mass,such as the electron.
(Multiple Choice)
4.9/5  (36)
(36)
A device emits light at 761.7 nm.What is the frequency of this radiation?
(Multiple Choice)
4.7/5  (34)
(34)
What is the energy per mole of photons of light with a wavelength of 976.9 nm?
(Multiple Choice)
4.8/5  (36)
(36)
For which of the following electron transitions would a hydrogen atom emit a photon of the highest energy?
(Multiple Choice)
4.9/5  (28)
(28)
If the energy of 1.00 mole of photons is 245 kJ,what is the wavelength of the light?
(Multiple Choice)
4.8/5  (37)
(37)
If a cordless phone operates at a frequency of 9.00 108 s-1.What is the wavelength of this radiation?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following colors of visible light has the highest frequency?
(Multiple Choice)
4.8/5  (27)
(27)
Showing 41 - 60 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)