Exam 7: The Structure of Atoms
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
For which of the following transitions would a hydrogen atom emit the lowest energy photon?
(Multiple Choice)
4.8/5  (29)
(29)
Erwin Schrödinger developed a model for the behavior of electrons in atoms that is known as quantum mechanics.Which of the following statements concerning this model is/are CORRECT?
1)The energy of an electron is quantized.
2)The energy of an electron is equal to its mass multiplied by the square of its velocity.
3)Electrons travel in circular orbits around a nucleus.
(Multiple Choice)
4.8/5  (37)
(37)
The energy required to break one mole of hydrogen-hydrogen bonds in H2 is 436 kJ/mol.What is the longest wavelength of light capable of breaking a single H-H bond?
(Multiple Choice)
4.8/5  (35)
(35)
The Bohr model predicts that the energy of an atom's electron is ________,meaning that the electron can only occupy orbitals of specific energies.
(Short Answer)
4.8/5  (38)
(38)
Which of the following properties is associated with the value of the 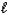 quantum number?
quantum number?
(Multiple Choice)
4.9/5  (37)
(37)
Occupied states or energy levels in the hydrogen atom with n > 1 are called _____ states.
(Short Answer)
4.8/5  (39)
(39)
A point in a standing wave that has zero amplitude is called a(n)________.
(Short Answer)
4.8/5  (35)
(35)
How many values are there for the magnetic quantum number ( 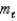 )when the value of the angular momentum quantum number (
)when the value of the angular momentum quantum number ( 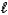 )is 4?
)is 4?
(Multiple Choice)
4.8/5  (41)
(41)
A red laser pointer emits light at a wavelength of 488 nm.If the laser emits 7.5 10-4 J of energy per second in the form of visible radiation,how many photons per second are emitted from the laser?
(Multiple Choice)
4.9/5  (45)
(45)
According to the Bohr model for the hydrogen atom,the energy necessary to excite an electron from n = 7 to n = 8 is ____ the energy necessary to excite an electron from n = 5 to n = 6.
(Multiple Choice)
4.7/5  (38)
(38)
In Bohr's atomic theory,when an electron moves from one energy level to another energy level more distant from the nucleus,
(Multiple Choice)
4.7/5  (40)
(40)
Which type of experiment demonstrates that light has the properties of a particle?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements is/are CORRECT?
1)The angular momentum quantum number, 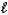 ,of an electron in an s orbital is always equal to 0.
2)The magnetic quantum number,
,of an electron in an s orbital is always equal to 0.
2)The magnetic quantum number, 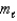 ,of an electron in a p orbital is +1,0,or -1.
3)The principle quantum number,n,of an electron in a d orbital must be equal to or greater than 3.
,of an electron in a p orbital is +1,0,or -1.
3)The principle quantum number,n,of an electron in a d orbital must be equal to or greater than 3.
(Multiple Choice)
4.9/5  (41)
(41)
A device operates at a frequency of 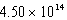 Hz.What is the wavelength of this radiation?
Hz.What is the wavelength of this radiation?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following sets of quantum numbers refers to a 4d orbital?
(Multiple Choice)
5.0/5  (37)
(37)
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes a transition from level
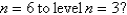 (
( 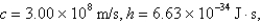
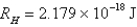 )
)
(Multiple Choice)
4.9/5  (49)
(49)
Which of the following statements is/are CORRECT?
1)A paramagnetic substance is attracted to a magnetic field.
2)An atom with no unpaired electrons is ferromagnetic.
3)Atoms with one or more unpaired electrons are paramagnetic.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 21 - 40 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)