Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. 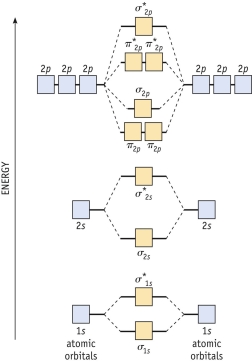 -Refer to diagram 9-1.Identify the molecule with the shortest bond length.
-Refer to diagram 9-1.Identify the molecule with the shortest bond length.
(Multiple Choice)
4.7/5  (38)
(38)
How many sigma ( )bonds and pi ( )bonds are in carbon monoxide?
(Multiple Choice)
4.8/5  (40)
(40)
When an atom in a molecule or ion is described as sp3 hybridized,its electron pair geometry is
(Multiple Choice)
4.9/5  (39)
(39)
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules.  -Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
(Multiple Choice)
4.8/5  (37)
(37)
Upon combustion,ethene (C2H4)is converted to carbon dioxide and water.What change in the hybridization of carbon occurs in this reaction?
(Multiple Choice)
4.9/5  (44)
(44)
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules.  -Refer to Diagram 9-1.What is the molecular orbital configuration of F2?
-Refer to Diagram 9-1.What is the molecular orbital configuration of F2?
(Multiple Choice)
4.8/5  (40)
(40)
What is the molecular geometry around a central atom that is sp2 hybridized,has three sigma bonds,and one pi bond?
(Multiple Choice)
4.8/5  (31)
(31)
What is the molecular geometry around a central atom that is sp3 hybridized and has one lone pair of electrons?
(Multiple Choice)
4.7/5  (33)
(33)
Atomic orbitals combine most effectively to form molecular orbitals when
(Multiple Choice)
4.8/5  (40)
(40)
Benzene,C6H6,consists of a six member ring of sp2 hybridized carbon atoms.Each carbon atom has one unhybridized p orbital.How many 2p bonding,antibonding,and nonbonding molecular orbitals exist for benzene?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following statements concerning valence bond (VB)theory is/are CORRECT?
1)VB theory can describe molecular bonding in excited states.
2)VB theory assumes that electrons are localized between pairs of atoms.
3)VB theory predicts localized lone pairs of electrons.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following statements concerning molecular orbital (MO)bond theory is/are CORRECT?
1)MO theory can describe molecular bonding in excited states.
2)Molecular orbitals are obtained from the combination of atomic orbitals.
3)MO theory predicts that electrons are delocalized over the molecule through molecular orbitals.
(Multiple Choice)
4.8/5  (34)
(34)
How many sigma and pi bonds are in the molecule pictured below? 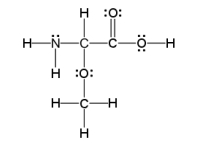
(Multiple Choice)
4.8/5  (45)
(45)
What is the maximum number of hybridized orbitals that can be formed by a fluorine atom?
(Multiple Choice)
4.9/5  (40)
(40)
Which molecule will have the following valence molecular orbital level energy diagram?
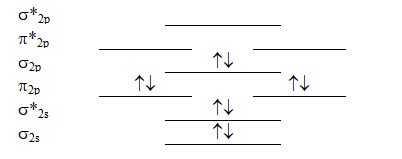
(Multiple Choice)
4.7/5  (46)
(46)
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules.  -Refer to Diagram 9-1.What is the molecular orbital configuration of N22+?
-Refer to Diagram 9-1.What is the molecular orbital configuration of N22+?
(Multiple Choice)
5.0/5  (39)
(39)
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s).For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules.  -Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be paramagnetic?
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be paramagnetic?
(Multiple Choice)
4.7/5  (28)
(28)
How many sigma ( )bonds and pi ( )bonds are in the following molecule? 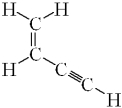
(Multiple Choice)
4.7/5  (29)
(29)
Showing 41 - 60 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)