Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
Which cycloalkane has the greatest ring strain per-CH2-unit?
(Multiple Choice)
5.0/5  (38)
(38)
Dimethyl sulfoxide (DMSO)is a polar aprotic solvent that is frequently used for organic reactions.Rank the following sodium halide salts for decreasing solubility in DMSO.
(Multiple Choice)
4.9/5  (39)
(39)
What is the VSEPR geometry for the carbon atom of a carbonyl?
(Multiple Choice)
4.8/5  (40)
(40)
Rank 1,4-dimethylbenzene,phenol,and N,N-dimethylaniline in order of decreasing solubility in the organic solvent toluene.
(Essay)
4.8/5  (33)
(33)
Which functional group will engage in dipole-dipole interactions,but will not serve as a hydrogen-bond acceptor?
(Multiple Choice)
4.7/5  (34)
(34)
Are any of the four 1,2-difluorocyclopropane isomers drawn below polar? Indicate the direction of the net molecular dipole,if one is present. 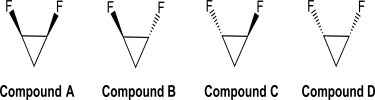
(Essay)
4.9/5  (35)
(35)
Consider the structure of sodium benzoate,NaOC(O)Ph,the sodium salt of benzoic acid.In which of the following solvents would you predict sodium benzoate to be soluble?
IWater,H2O
IIPentane,CH3(CH2)3CH3
IIIDiethyl ether,(CH3CH2)2O
IVMethanol,CH3OH
VAcetone,CH3C(O)CH3
(Multiple Choice)
4.9/5  (51)
(51)
When mixed,which of the following pairs of compounds will exhibit both ion-dipole and ion-ion intermolecular attractive forces? 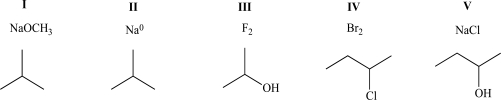
(Multiple Choice)
4.7/5  (37)
(37)
How does the presence of the lone pair affect the geometry of the central atom in the following molecule? 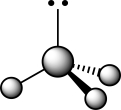 I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following molecules contains a nitrogen atom that has bent molecular geometry? 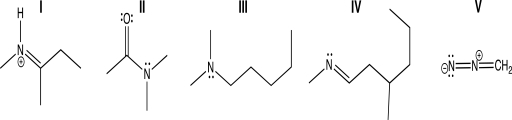
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following molecules possesses at least one polar covalent bond but does not have an overall net molecular dipole?
(Multiple Choice)
4.7/5  (35)
(35)
How many hydrogen-bond donors and acceptors are present in the following molecule? 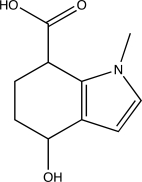
(Multiple Choice)
5.0/5  (36)
(36)
What is the strongest intermolecular attractive force between an alcohol and a ketone?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 41 - 53 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)