Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
Which of the following molecules contains a nitrogen atom with linear geometry? 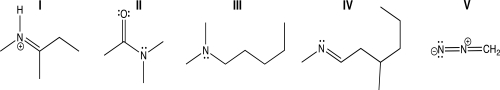
(Multiple Choice)
4.9/5  (38)
(38)
Which cycloalkane contains a CCC bond angle that deviates from the ideal tetrahedral bond angle by approximately 50°?
(Multiple Choice)
4.9/5  (38)
(38)
Sodium chloride,an ionic compound,is highly water soluble but minimally soluble in the polar aprotic solvent dimethyl sulfoxide (DMSO).Why?
(Essay)
4.9/5  (38)
(38)
Add substituents using dash-wedge notation to achieve the structure specified.
(a) An alkene that has a fluorine atom pointing back on the leftmost carbon and a methyl group coming out on the rightmost carbon.Assume that hydrogen fills the valence of carbon.
(b) A tetrahedral carbon with two chlorine atoms pointing down on the plane of the paper,a bromine atom pointing up,and a hydrogen atom pointing back.
(Short Answer)
4.9/5  (45)
(45)
Which of the following benzene derivatives would be most soluble in benzene? Why? 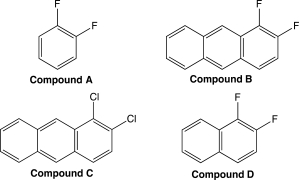
(Essay)
4.8/5  (37)
(37)
What is the strongest intermolecular attractive force possible between an alkyl chloride and an alkane?
(Multiple Choice)
4.7/5  (25)
(25)
Rank N-N-dimethylaniline,phenethylamine,and phenethylamine hydrochloride in order of decreasing boiling point.Explain your reasoning.
(Essay)
4.8/5  (46)
(46)
Which cycloalkane contains a CCC bond angle that deviates from the ideal tetrahedral bond angle by approximately 20°?
(Multiple Choice)
4.8/5  (43)
(43)
The solvent tert-butyl methyl ether (MTBE)is used as a "greener" replacement for the organic solvent diethyl ether,because it has less propensity to form peroxides upon standing.Draw the line structures of both diethyl ether and MTBE.Look up the physical properties (boiling point,flash point)of both solvents.Explain why the boiling point of diethyl ether is lower.
(Essay)
4.9/5  (39)
(39)
What is the VSEPR geometry for any carbon atom in a phenyl ring?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following intermolecular forces is responsible for the boiling-point trends in alkanes?
(Multiple Choice)
4.9/5  (39)
(39)
Propanol can be dissolved in diethyl ether and treated with sodium hydride,a base,to form a sodium alkoxide,as shown below. 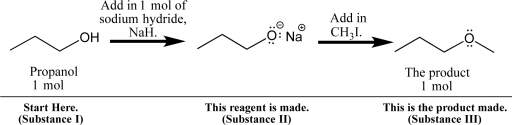 Although this and related chemical reactions will be studied in later chapters,you can apply your knowledge of solubility to the process.
Fill in your solubility predictions using the table below.Do you expect propanol (I),sodium propoxide (II),and the new ether product (III)to be soluble in the reaction solvent,diethyl ether? If soluble,predict the intermolecular interactions that will exist in solution.Also,anticipate the physical appearance of the reaction at each point: Will the solution be transparent,or will a precipitate form?
Although this and related chemical reactions will be studied in later chapters,you can apply your knowledge of solubility to the process.
Fill in your solubility predictions using the table below.Do you expect propanol (I),sodium propoxide (II),and the new ether product (III)to be soluble in the reaction solvent,diethyl ether? If soluble,predict the intermolecular interactions that will exist in solution.Also,anticipate the physical appearance of the reaction at each point: Will the solution be transparent,or will a precipitate form?

(Short Answer)
4.8/5  (38)
(38)
Tertiary amides are typically insoluble in water.The solvent dimethyl acetamide,CH3CON(CH3)2,which is commonly called DMA,is an exception.Explain why the solvent DMA is soluble in water.Diagram the intermolecular forces that make DMA soluble in water.
(Short Answer)
4.8/5  (32)
(32)
Which of the following choices correctly describes the structure of the ball-and-stick representation with the formula H3C+? 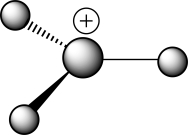
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following molecules contains a trigonal planar nitrogen atom connected to two different tetrahedral carbon atoms? 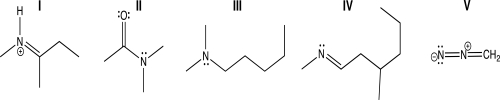
(Multiple Choice)
4.8/5  (38)
(38)
Your lab partner disobeyed lab rules and poured table salt from lunch into your unknown organic white powder,which is aprotic and contains an ester functional group.What solvent might you add to the solid mixture to remove the table salt,leaving the ester? What solvent is appropriate to remove the unknown ester,leaving instead the salt? Explain your reasoning.
(Essay)
4.8/5  (32)
(32)
When applying VSEPR theory to determine the geometry about a central atom,it is important to count the total number of bonded and nonbonded electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many bonded electron groups must be considered for each of these central atoms? 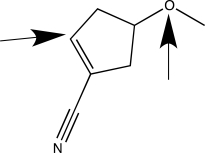
(Multiple Choice)
4.8/5  (34)
(34)
Turn the original molecule shown below 90° in a clockwise direction on the plane of this paper.Which choice represents the product of this manipulation? 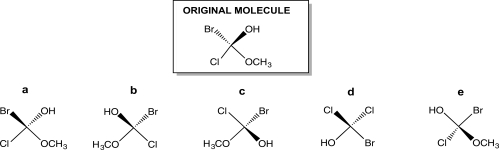
(Multiple Choice)
5.0/5  (33)
(33)
Showing 21 - 40 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)