Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
Why is a σ bond formed from the overlap of two sp2 orbitals stronger than one formed from the end-on-end overlap of two p orbitals?
(Essay)
4.8/5  (28)
(28)
Sketch an orbital picture of ethylene,H2C  CH2,and highlight the orbitals that overlap to form each σ and π bond.Identify the orbitals from each atom that overlap to form the σ and π bonds.Why is the CC π bond weaker than the CC σ bond? Use orbital structure as part of your explanation.
CH2,and highlight the orbitals that overlap to form each σ and π bond.Identify the orbitals from each atom that overlap to form the σ and π bonds.Why is the CC π bond weaker than the CC σ bond? Use orbital structure as part of your explanation.
(Essay)
4.8/5  (40)
(40)
Which CC bond in the molecule below is formed from overlap of two 2p orbitals? 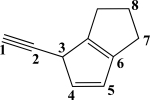
(Multiple Choice)
4.8/5  (45)
(45)
Consider the hybrid structure below and the labeling scheme provided.Draw the most stable resonance contributor.Identify the hybridization and VSEPR geometry of each atom. 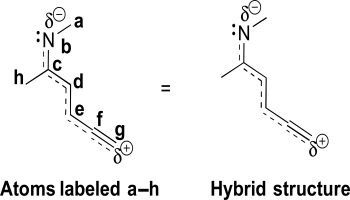
(Essay)
4.8/5  (44)
(44)
For one or more of the following molecules,two distinct configurations about the double bond are possible,and the two alkyl substituents are trans.Select the molecule or molecules that fit this description. 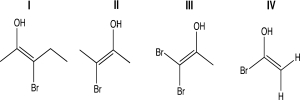
(Multiple Choice)
4.8/5  (30)
(30)
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals would carbon use for a CO π bond? 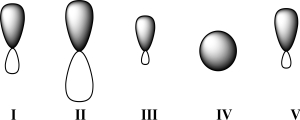
(Multiple Choice)
4.7/5  (41)
(41)
Compare the p orbital orientation and overlap needed to form a σ bond and a π bond.Using an example,create a diagram for each type of bond,and differentiate between σ and π bond characteristics.
(Short Answer)
5.0/5  (42)
(42)
Which two atomic orbitals overlap to form the MOs of σ symmetry that correspond to the CC bond in ethylene?
(Multiple Choice)
5.0/5  (45)
(45)
Which two carbon atoms participate in the strongest σ bond? 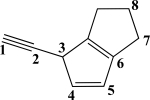
(Multiple Choice)
4.9/5  (33)
(33)
How many total electrons reside in molecular orbitals of π symmetry for this molecule? 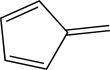
(Multiple Choice)
4.8/5  (27)
(27)
Tubulysin D is a peptide-based marine natural product with biological activity.Identify the following structural features of tubulysin D.
(a) Place a box around any sp3 nitrogen.
(b) Star (*)any oxygen atom that has two oxygen lone pairs in sp2-hybridized orbitals.
(c) Place a number symbol (#)on any oxygen atom that is sp2 hybridized as a consequence of having a lone pair in a 2p orbital.
(d) Use an arrow to point to any N atom that has trigonal planar geometry. 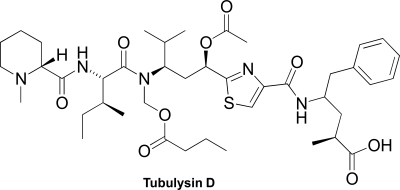
(Short Answer)
4.8/5  (37)
(37)
Which of the following functional groups has a lone pair residing in an sp-hybridized orbital?
(Multiple Choice)
4.8/5  (34)
(34)
Which characteristics describe the bonding MO for the weakest individual covalent bond in ethene,C2H4?
I. The MO possesses π symmetry.
II.The MO possesses σ symmetry.
III.The MO can be called the HOMO.
IV.The MO can be called the LUMO.
V.The MO contains two electrons of opposing spin.
(Multiple Choice)
4.7/5  (33)
(33)
Amide bonds join amino acids together to make proteins.Sketch an orbital picture of the amide functional group given below.Identify the orbitals from each atom that are used to form the σ and π bonds in the given amide.Which orbitals house the lone pairs on N and O? 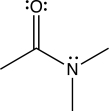
(Essay)
4.8/5  (39)
(39)
Given below are 2s,2p,sp,sp2,and sp3 orbitals,all drawn to scale and in no particular order.Which of these orbitals represents the hybrid orbital with the greatest effective electronegativity? 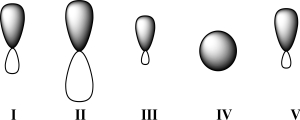
(Multiple Choice)
4.8/5  (50)
(50)
Given below are 2s,2p,sp,sp2,and sp3 orbitals,in random order.Which of these orbitals exhibits 33% s character? 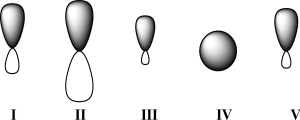
(Multiple Choice)
4.9/5  (35)
(35)
Which CC bond in the molecule below is formed from overlap of an sp3 orbital and an sp2 orbital? 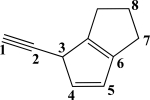
(Multiple Choice)
5.0/5  (43)
(43)
What abbreviation is used to designate the molecular orbital of highest energy that is occupied with electrons?
(Multiple Choice)
4.9/5  (39)
(39)
Draw line structures of a molecule with the formula C5H11N that has the characteristics outlined in (a),(b),and (c)below.
(a) An sp2 nitrogen atom with a conjugated lone pair,one methyl substituent,and one ethyl substituent
(b) A disubstituted trans alkene and a primary amine with an sp3 nitrogen connected to an sp3 carbon
(c) A three-membered ring containing a secondary sp3 nitrogen with cis methyl and ethyl substituents on the ring
(Short Answer)
4.8/5  (42)
(42)
Showing 21 - 40 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)