Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
An alkene contains a double bond.Why is the C  C of an alkene rigid and unable to freely rotate?
C of an alkene rigid and unable to freely rotate?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following statements about molecular orbital theory are true?
I. According to MO theory,electrons are localized on specific atoms.
II.The linear combination of atomic orbitals method is used to generate molecular orbitals for a molecule.
III. The number of molecular orbitals created from the LCAO approach is identical to the number of atomic orbitals that were originally combined.
IV. Molecular orbital theory is a powerful bonding theory that accurately predicts structures of complex molecules.
V. The MOs generated are classified as either bonding,nonbonding,or antibonding.
(Multiple Choice)
4.8/5  (33)
(33)
From left to right,identify the hybridization of the three carbon atoms in the interesting organic structure below.These interesting structures are called cumulenes. 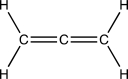
(Multiple Choice)
4.8/5  (36)
(36)
Acetonitrile,C2H3N,is a polar aprotic solvent commonly used in organic reactions.Draw the structure of acetonitrile.Determine the number of σ bonds,the number of π bonds,and the number of electrons occupying nonbonding MOs for the molecule.
(Short Answer)
4.9/5  (36)
(36)
What is the geometry and hybridization of the central carbon atom in the cumulene below? 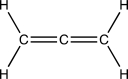
(Multiple Choice)
4.7/5  (24)
(24)
According to valence bond theory,which atomic orbitals of carbon may be hybridized to account for bonding? Why?
(Multiple Choice)
4.8/5  (38)
(38)
Naltrexone is an antagonist at the mu opioid receptor.What is the hybridization state and geometry of the nitrogen atom in naltrexone? 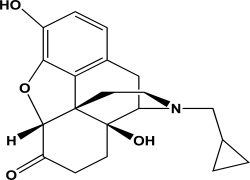
(Multiple Choice)
4.8/5  (37)
(37)
What abbreviation is used to designate the lowest-energy molecular orbital for a molecule that is devoid of electrons?
(Multiple Choice)
4.8/5  (40)
(40)
Using line structures,deduce individual resonance contributors from the resonance hybrid structure given here.Identify any lone pairs that are localized,rather than delocalized.Based on orbital hybridization theory,what orbitals accommodate these lone pairs? 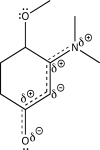
(Short Answer)
4.8/5  (37)
(37)
Which of the following structures contains an sp2-hybridized oxygen atom because a lone pair on oxygen is delocalized via resonance? 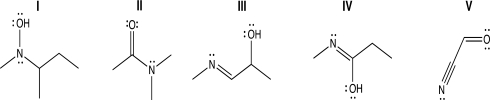
(Multiple Choice)
4.9/5  (33)
(33)
Bioymifi is a novel small molecule that selectively triggers programmed cell death in certain cancer cells.Identify the following structural features in Bioymifi: (a)phenyl ring,(b)a lone pair found in a 2p orbital that can be delocalized three different ways,(c)an sp2-hybridized oxygen whose lone pairs are not delocalized,(d)an sp2-hybridized oxygen whose lone pairs are delocalized,(e)a nitrogen with bent geometry,and (f)a nitrogen with trigonal planar geometry. 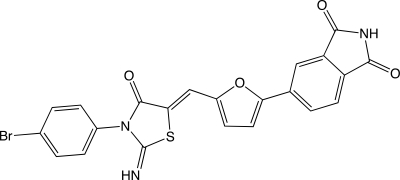
(Short Answer)
4.8/5  (31)
(31)
Which CC bond in the molecule below is formed from overlap of two sp2-hybridized orbitals? 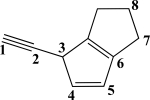
(Multiple Choice)
4.9/5  (31)
(31)
Use your knowledge of hybridization to fill in the table below.Rank the sigma bonds labeled A,B,C,and D from strongest to weakest.Explain your answer. 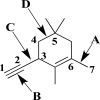
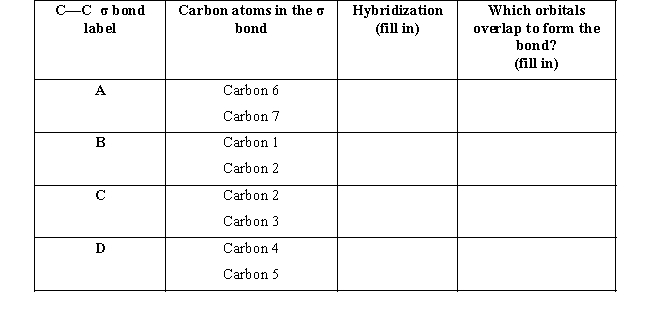
(Essay)
4.8/5  (39)
(39)
Rank the highlighted σ bonds in order of decreasing length. 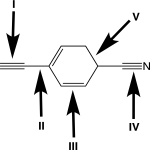
(Multiple Choice)
4.8/5  (35)
(35)
For one or more of the following molecules,(1)two distinct configurations about the double bond are possible,and (2)the -OH and -Br are trans.Select the molecule or molecules that fit this description. 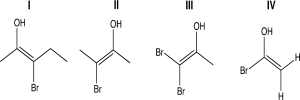
(Multiple Choice)
4.9/5  (30)
(30)
Showing 41 - 56 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)