Exam 1: Remembering General Chemistry: Electronic Structure and Bonding
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding90 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry43 Questions
Exam 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure136 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space125 Questions
Exam 5: Alkenes: Structure,nomenclature,and an Introduction to Reactivity - Thermodynamics and Kinetics84 Questions
Exam 6: The Reactions of Alkenes - the Stereochemistry of Addition Reactions89 Questions
Exam 7: The Reactions of Alkynes - Introduction to Multistep Synthesis124 Questions
Exam 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene185 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides228 Questions
Exam 10: Reactions of Alcohols, ethers, epoxides, amines and Sulfur-Containing Compounds109 Questions
Exam 11: Organometallic Compounds65 Questions
Exam 12: Radicals141 Questions
Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy140 Questions
Exam 14: Nmr Spectroscopy122 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives126 Questions
Exam 16: Reactions of Aldehydes and Ketones122 Questions
Exam 17: Reactions at the Α-Carbon121 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes168 Questions
Exam 19: More About Amines - Reactions of Heterocylic Compounds126 Questions
Exam 20: The Organic Chemistry of Carbohydrates110 Questions
Exam 21: Amino Acids,peptides,and Proteins117 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions92 Questions
Exam 23: The Organic Chemistry of the Coenzymes, compounds Derived From Vitamins102 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways90 Questions
Exam 25: The Organic Chemistry of Lipids37 Questions
Exam 26: The Chemistry of the Nucleic Acids94 Questions
Exam 27: Synthetic Polymers116 Questions
Exam 28: Pericyclic Reactions102 Questions
Select questions type
Triethylamine [(CH3CH2)3N] is a molecule in which the nitrogen atom is ________ hybridized and the CNC bond angle is ________.
(Multiple Choice)
4.8/5  (25)
(25)
Draw the structure of a molecule which contains only carbon and hydrogen atoms (only three of which are carbon)and in which two of the carbons are sp2 hybridized and the other is sp hybridized.
(Essay)
4.8/5  (48)
(48)
Expand the condensed structure below to show the covalent bonds and the lone-pair electrons.
(CH3)2CHCH2CHO
(Essay)
4.8/5  (43)
(43)
Propanal is a compound detected on the surface of comet 67P by the Philae Lander.How many hydrogen atoms are present at the indicated carbon? 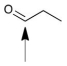
(Multiple Choice)
4.7/5  (34)
(34)
The structure of a widely used anesthetic propofol is given below.How many sp3 hybridized atoms are in this molecule? 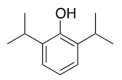
(Multiple Choice)
4.8/5  (41)
(41)
Draw the Kekulé structure and show the direction of the dipole moment for CH2Cl2.
(Essay)
4.8/5  (28)
(28)
Which of the following structures,including formal charges,is correct for diazomethane,CH2N2?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following species have tetrahedral bond angles? 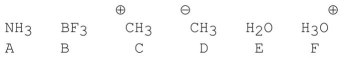
(Multiple Choice)
4.9/5  (31)
(31)
Identify the hybridization of the nitrogen atom in the molecule (CH3)3N.
(Short Answer)
4.9/5  (43)
(43)
Showing 81 - 90 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)