Exam 1: Remembering General Chemistry: Electronic Structure and Bonding
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding90 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry43 Questions
Exam 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure136 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space125 Questions
Exam 5: Alkenes: Structure,nomenclature,and an Introduction to Reactivity - Thermodynamics and Kinetics84 Questions
Exam 6: The Reactions of Alkenes - the Stereochemistry of Addition Reactions89 Questions
Exam 7: The Reactions of Alkynes - Introduction to Multistep Synthesis124 Questions
Exam 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene185 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides228 Questions
Exam 10: Reactions of Alcohols, ethers, epoxides, amines and Sulfur-Containing Compounds109 Questions
Exam 11: Organometallic Compounds65 Questions
Exam 12: Radicals141 Questions
Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy140 Questions
Exam 14: Nmr Spectroscopy122 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives126 Questions
Exam 16: Reactions of Aldehydes and Ketones122 Questions
Exam 17: Reactions at the Α-Carbon121 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes168 Questions
Exam 19: More About Amines - Reactions of Heterocylic Compounds126 Questions
Exam 20: The Organic Chemistry of Carbohydrates110 Questions
Exam 21: Amino Acids,peptides,and Proteins117 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions92 Questions
Exam 23: The Organic Chemistry of the Coenzymes, compounds Derived From Vitamins102 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways90 Questions
Exam 25: The Organic Chemistry of Lipids37 Questions
Exam 26: The Chemistry of the Nucleic Acids94 Questions
Exam 27: Synthetic Polymers116 Questions
Exam 28: Pericyclic Reactions102 Questions
Select questions type
Several volatile compounds are responsible for the aroma of plums.One of these compounds is linalool whose a skeletal structure is shown below.Convert it into Kekulé structure. 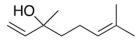
(Essay)
4.7/5  (42)
(42)
How many sp2 hybridized carbons are present in allene (H2C=C=CH2)?
(Multiple Choice)
4.8/5  (26)
(26)
Draw the Kekulé structure for each of the following:
a.CH3CH2OH b.CH3CHO c.(CH3)3C+
(Essay)
4.8/5  (25)
(25)
The lone-pair electrons of the methyl anion occupy a(n)________ orbital.
(Multiple Choice)
4.9/5  (43)
(43)
Identify the compound(s)that have a nonzero dipole moment.You may choose more than one answer.
(Multiple Choice)
4.7/5  (42)
(42)
In 2015,the European Space Agency's Philae Lander detected the presence of methylisocyanate CH3NCO on the comet 67P.Draw the Lewis structure of this compound,showing all lone pairs.
(Essay)
4.7/5  (35)
(35)
Both sigma (σ)and pi (π)bonds can be formed by overlapping p orbitals.Describe the difference.
(Essay)
4.9/5  (36)
(36)
What kind of molecular orbital (σ,σ*,π,or π*)results when the two atomic orbitals shown below interact in the manner indicated? 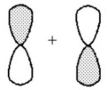
(Short Answer)
4.8/5  (41)
(41)
Which of the following statements correctly describes the third electron shell that surrounds the nucleus of an atom?
(Multiple Choice)
4.9/5  (31)
(31)
Nitrous oxide N2O,also known as laughing gas is often used in surgery and dentistry for its anesthetic and analgesic effects.Draw its Lewis structure?
(Essay)
5.0/5  (40)
(40)
What kind of molecular orbital (σ,σ*,π,or π*)results when the two atomic orbitals shown below interact in the manner indicated? 
(Short Answer)
4.9/5  (28)
(28)
Provide the mathematical equation for the dipole moment of a bond,and identify the variables.
(Essay)
4.9/5  (37)
(37)
Several volatile compounds are responsible for the aroma of plums.One of these compounds is γ-decalactone whose Kekulé structure is shown below.Convert it into a skeletal structure. 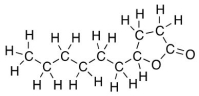
(Essay)
4.8/5  (33)
(33)
Which of the following molecules does not exhibit a net dipole moment of zero?
(Multiple Choice)
4.8/5  (33)
(33)
The compound methylamine,CH3NH2,contains a C-N bond.In this bond,which of the following best describes the charge on the nitrogen atom?
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following is the most likely electronic structure for C2H2?
(Multiple Choice)
4.9/5  (40)
(40)
What orbitals overlap to create the C-H bond in ethene (H2C=CH2)?
(Multiple Choice)
4.9/5  (42)
(42)
In what type of orbital are the lone pair electrons of methoxide (CH3O-)found ?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)