Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding90 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry43 Questions
Exam 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure136 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space125 Questions
Exam 5: Alkenes: Structure,nomenclature,and an Introduction to Reactivity - Thermodynamics and Kinetics84 Questions
Exam 6: The Reactions of Alkenes - the Stereochemistry of Addition Reactions89 Questions
Exam 7: The Reactions of Alkynes - Introduction to Multistep Synthesis124 Questions
Exam 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene185 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides228 Questions
Exam 10: Reactions of Alcohols, ethers, epoxides, amines and Sulfur-Containing Compounds109 Questions
Exam 11: Organometallic Compounds65 Questions
Exam 12: Radicals141 Questions
Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy140 Questions
Exam 14: Nmr Spectroscopy122 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives126 Questions
Exam 16: Reactions of Aldehydes and Ketones122 Questions
Exam 17: Reactions at the Α-Carbon121 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes168 Questions
Exam 19: More About Amines - Reactions of Heterocylic Compounds126 Questions
Exam 20: The Organic Chemistry of Carbohydrates110 Questions
Exam 21: Amino Acids,peptides,and Proteins117 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions92 Questions
Exam 23: The Organic Chemistry of the Coenzymes, compounds Derived From Vitamins102 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways90 Questions
Exam 25: The Organic Chemistry of Lipids37 Questions
Exam 26: The Chemistry of the Nucleic Acids94 Questions
Exam 27: Synthetic Polymers116 Questions
Exam 28: Pericyclic Reactions102 Questions
Select questions type
Which of the following functional groups will exhibit no IR absorption at 1630 - 1780 cm-1 or at 3200 - 3550 cm-1?
(Multiple Choice)
4.9/5  (31)
(31)
An infrared wavelength of 4.48μm is equivalent to a wavenumber of ________ cm-1.
(Short Answer)
5.0/5  (32)
(32)
Which of the following m/z values is least intense in the mass spectrum of 1-chloropropane?
(Multiple Choice)
4.9/5  (35)
(35)
Which has the higher speed in a vacuum,ultraviolet or infrared light?
(Short Answer)
4.9/5  (36)
(36)
While cleaning the organic stockroom,a student found a bottle of ethanol labeled "Denatured with Benzene." He decided to determine the concentration of benzene in the ethanol by acquiring the UV spectrum of the liquid in a 2.0 cm cell.The spectrum exhibited an absorption band at 260 nm which was attributable to benzene,and the absorbance of this band was 0.69.In a reference source,the student found that the molar absorptivity of this benzene band in ethanol was 230 M-1cm-1.Show how the student calculated the concentration of the benzene in the ethanol.
(Essay)
5.0/5  (24)
(24)
Which of the following is/are true about the MS of 1-bromobutane?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following m/z values is the base peak for benzyl alcohol? 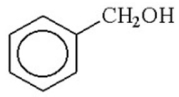
(Multiple Choice)
4.9/5  (39)
(39)
Which of the infrared regions is considered to be the fingerprint region?
(Multiple Choice)
4.9/5  (34)
(34)
Which compound's carbonyl stretch occurs at the greatest wavenumber?
(Multiple Choice)
4.8/5  (37)
(37)
Which compound would be expected to show intense IR absorption at 1715 cm-1?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following statements best explains the information we can gain from mass spectrometry?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is the base peak for the compound below? 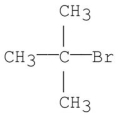
(Multiple Choice)
4.8/5  (35)
(35)
Which region of the electromagnetic spectrum,IR or UV,contains photons of the higher energy?
(Short Answer)
4.8/5  (37)
(37)
Which region of the electromagnetic spectrum,IR or X-ray,is characterized by waves of lower frequency?
(Short Answer)
5.0/5  (39)
(39)
What technique can be used to determine the molecular formula of a compound?
(Short Answer)
4.8/5  (26)
(26)
Which of the following carbon-hydrogen bonds exhibits the lowest wavenumber for a C-H stretch in infrared spectroscopy? 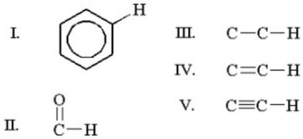
(Multiple Choice)
4.8/5  (29)
(29)
Give the fragment(s)that corresponds to a m/z charge of 58 for ethylpropylamine.You may choose more than one answer.
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following structures will give a base peak of 43 in mass spectrometry?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)