Exam 24: Nuclear Reactions and Their Applications
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Write a complete, balanced equation to represent the alpha decay of radon-210.
Free
(Essay)
4.7/5  (33)
(33)
Correct Answer:
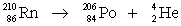
Which one of the following is a subatomic particle closely related to the positron?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
B
The radiochemist, Will I. Glow, studied thorium-232 and found that 2.82 × 10-7 moles emitted 8.42 × 106 particles in one year. What is the decay constant for thorium-232?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
B
A bottle of vintage red wine has lost its label. The concentration of tritium ( 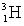 ) in the wine is 0.34 times that found in freshly bottled wines. If the half-life of tritium is 12.3 years, estimate the time elapsed since the wine was bottled.
) in the wine is 0.34 times that found in freshly bottled wines. If the half-life of tritium is 12.3 years, estimate the time elapsed since the wine was bottled.
(Short Answer)
4.9/5  (35)
(35)
A patient's thyroid gland is to be exposed to an average of 5.5 Ci for 16 days as an ingested sample of iodine-131 decays. If the energy of the radiation is 9.7 × 10-14 J and the mass of the thyroid is 32.0 g, what is the dose received by the patient?
(Multiple Choice)
4.8/5  (47)
(47)
Calcium-39 undergoes positron decay. Each positron carries 5.49 MeV of energy. How much energy will be emitted when 0.0025 mol of calcium-39 decays?
(Multiple Choice)
4.8/5  (43)
(43)
Which one of the following descriptions relating to nuclear reactions is correct?
(Multiple Choice)
4.7/5  (37)
(37)
What is the mechanism by which control rods slow down the fission rate in a nuclear reactor?
(Essay)
4.8/5  (36)
(36)
When an electron and its anti-particle, a positron, collide, they annihilate each other. Calculate the energy released in this process, in J. (The positron mass is the same as the electron mass, namely 9.11 × 10-31 kg.)
(Short Answer)
4.9/5  (38)
(38)
Calculate to four significant figures
a. the mass defect in kg, and
b. the energy released in kJ/mol, when a neutron decays to produce a proton and an electron. The neutron, proton, and electron masses are 1.67493 × 10-27 kg, 1.67262 × 10-27 kg and 9.10939 × 10-31 kg, respectively.
(Essay)
4.8/5  (31)
(31)
Which of the following isotopes is most likely to be unstable?
(Multiple Choice)
4.8/5  (29)
(29)
Select the nuclide that completes the following nuclear reaction. 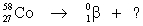
(Multiple Choice)
4.7/5  (40)
(40)
Sodium-21 will emit positrons each having an energy of 4.0 × 10-13 J. What is this energy in MeV?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following isotopes is most likely to be unstable?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following series of radioactive decays would convert Pa-234 to Ra-226?
(Multiple Choice)
4.8/5  (38)
(38)
A 9.52 × 10-5 mol sample of rubidium-86 emits 8.87 × 1016 particles in one hour. What is the half-life of rubidium-86?
(Multiple Choice)
5.0/5  (34)
(34)
Exposure to 10 nCi for 10 minutes is more hazardous for a child than for an adult because
(Multiple Choice)
4.8/5  (32)
(32)
What is the specific activity (in Ci/g) of an isotope if 3.56 mg emits 4.26 × 108 particles per second?
(Multiple Choice)
4.9/5  (37)
(37)
Of the naturally-occurring elements on earth today, identify by their chemical symbols
a. two which would have resulted directly from the "big bang".
b. one which can only be formed in supernova explosions.
c. two which are formed during the normal life of first generation stars.
(Essay)
4.7/5  (38)
(38)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)