Exam 21: Electrochemistry: Chemical Change and Electrical Work
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
In a fuel cell, an external source of electrical power is used to drive a non-spontaneous reaction in which a fuel is produced.
Free
(True/False)
4.8/5  (32)
(32)
Correct Answer:
False
A primary battery is one which can be recharged.
Free
(True/False)
4.8/5  (39)
(39)
Correct Answer:
False
A concentration cell consists of two Zn/Zn2+ electrodes. The electrolyte in compartment A is 0.10 M Zn(NO3)2 and in compartment B is 0.60 M Zn(NO3)2. What is the voltage of the cell at 25°C?
Free
(Multiple Choice)
4.9/5  (26)
(26)
Correct Answer:
C
A concentration cell is based on the aqueous reaction
Cu2+(1.00 M) Cu2+(0.0100 M)
The cell consists of copper electrodes dipping into solutions of Cu2+ ions. The anions present are sulfate ions. Draw a neat diagram to represent this cell, showing and labeling all necessary components including: anode, cathode, electron flow, cation flow, and anion flow.
(Essay)
5.0/5  (31)
(31)
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for zinc will be _____. Zn(s) + ReO4-(aq) Re(s) + Zn2+(aq) (acidic solution)
(Multiple Choice)
4.8/5  (28)
(28)
Examine the following half-reactions and select the strongest reducing agent among the species listed.
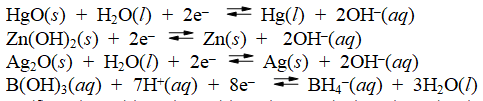
(Multiple Choice)
4.8/5  (43)
(43)
A concentration cell is based on the aqueous reaction
Cu2+(1.00 M) Cu2+(0.0100 M)
The cell consists of copper electrodes dipping into solutions of Cu2+ ions. The anions present are sulfate ions. Write the shorthand cell notation for this cell.
(Essay)
4.9/5  (24)
(24)
Predict the products of the cell reaction when a molten salt mixture of sodium bromide and calcium fluoride is electrolyzed (spectator ions are not considered to be products).
(Multiple Choice)
4.9/5  (32)
(32)
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for the iodide ion will be _____. I-(aq) + NO3-(aq) NO(g) + I2(s) (acidic solution)
(Multiple Choice)
4.8/5  (26)
(26)
a. Write a balanced equation to represent the overall reaction you would expect to occur in the electrolysis of molten KCl.
b. Write a balanced equation to represent the overall reaction you would expect to occur in the electrolysis of aqueous KCl.
c. Clearly explain why the products of the two processes are not the same.
(Essay)
4.9/5  (33)
(33)
Which one of the following pairs of substances could be used to construct a single redox electrode (i.e., they have an element in common, but in different oxidation states)?
(Multiple Choice)
4.8/5  (34)
(34)
Two cells are connected in series, so that the same current flows through two electrodes where the following half-reactions occur Cu2+(aq) + 2e- Cu(s) and Ag+(aq) + e- Ag(s)
For every 1.00 g of copper produced in the first process, how many grams of silver will be produced in the second one?
(Multiple Choice)
4.8/5  (38)
(38)
When the following redox equation is balanced with smallest whole number coefficients, the coefficient for nitrogen dioxide will be _____.
I2(s) + HNO3(aq) HIO3(aq) + NO2(g) + H2O(l)
(Multiple Choice)
4.8/5  (40)
(40)
Electrolytic cells utilize electrical energy to drive non-spontaneous redox reactions.
(True/False)
4.8/5  (25)
(25)
Calculate E°cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous.
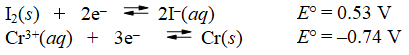 reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I-(aq)
reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I-(aq)
(Multiple Choice)
4.8/5  (36)
(36)
What is the value of the equilibrium constant for the cell reaction below at 25°C? E°cell = 0.61 V 2Cr(s) + 3Pb2+(aq)  3Pb(s) + 2Cr3+(aq)
3Pb(s) + 2Cr3+(aq)
(Multiple Choice)
4.7/5  (43)
(43)
Which of the following statements about voltaic and electrolytic cells is correct?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following elements could be prepared by electrolysis of the aqueous solution shown?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)