Exam 17: Equilibrium: the Extent of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
In water, the following equilibrium exists: H+(aq) + OH-(aq)  H2O(l)
In pure water at 25 °C, the concentration of H+ ions is 1.00 × 10-7 mol/L. Calculate the value of the equilibrium constant for the reaction as written above.
H2O(l)
In pure water at 25 °C, the concentration of H+ ions is 1.00 × 10-7 mol/L. Calculate the value of the equilibrium constant for the reaction as written above.
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
A
Although a system may be at equilibrium, the rate constants of the forward and reverse reactions will in general be different.
Free
(True/False)
4.9/5  (30)
(30)
Correct Answer:
True
A chemical reaction will reach equilibrium when the limiting reactant is used up.
Free
(True/False)
4.8/5  (32)
(32)
Correct Answer:
False
At a high temperature, the following reaction has an equilibrium constant of 1.0 × 102.
H2(g) + F2(g)  2HF(g)
If 1.00 mol of each of H2 and F2 are allowed to come to equilibrium in a 10.0 L vessel, calculate the equilibrium amounts of H2 and HF.
2HF(g)
If 1.00 mol of each of H2 and F2 are allowed to come to equilibrium in a 10.0 L vessel, calculate the equilibrium amounts of H2 and HF.
(Short Answer)
4.9/5  (34)
(34)
Consider the reversible reaction: 2NO2(g)  N2O4(g) If the concentrations of both NO2 and N2O4 are 0.016 mol L-1, what is the value of Qc?
N2O4(g) If the concentrations of both NO2 and N2O4 are 0.016 mol L-1, what is the value of Qc?
(Multiple Choice)
4.9/5  (34)
(34)
Write the mass-action expression, Qc , for the following chemical reaction. 3ClO2-(aq)  2ClO3-(aq) + Cl-(aq)
2ClO3-(aq) + Cl-(aq)
(Multiple Choice)
4.9/5  (42)
(42)
Unless H°rxn = 0, a change in temperature will affect the value of the equilibrium constant Kc.
(True/False)
4.8/5  (44)
(44)
Consider the equilibrium
H2(g) + Br2(g)  2HBr(g)
To a 20.0 L flask are added 0.100 moles of H2 and 0.200 moles of HBr. The equilibrium constant for this reaction is 989. Calculate the number of moles of Br2 in the flask when equilibrium is established. Make any reasonable approximation, clearly stating what that approximation is.
2HBr(g)
To a 20.0 L flask are added 0.100 moles of H2 and 0.200 moles of HBr. The equilibrium constant for this reaction is 989. Calculate the number of moles of Br2 in the flask when equilibrium is established. Make any reasonable approximation, clearly stating what that approximation is.
(Essay)
4.8/5  (40)
(40)
About half of the sodium carbonate produced is used in making glass products because it lowers the melting point of sand, the major component of glass. When sodium carbonate is added to water it hydrolyses according to the following reactions. CO32-(aq) + H2O(l)  HCO3-(aq) + OH-(aq) K1
HCO3-(aq) + H2O(l)
HCO3-(aq) + OH-(aq) K1
HCO3-(aq) + H2O(l)  H2CO3(aq) + OH-(aq) K2
These can be combined to yield
CO32-(aq) + 2H2O(l)
H2CO3(aq) + OH-(aq) K2
These can be combined to yield
CO32-(aq) + 2H2O(l)  H2CO3(aq) + 2OH-(aq) K3
What is the value of K3?
H2CO3(aq) + 2OH-(aq) K3
What is the value of K3?
(Multiple Choice)
4.9/5  (39)
(39)
At 850°C, the equilibrium constant Kp for the reaction C(s) + CO2(g)  2CO(g)
Has a value of 10.7. If the total pressure in the system at equilibrium is 1.000 atm, what is the partial pressure of carbon monoxide?
2CO(g)
Has a value of 10.7. If the total pressure in the system at equilibrium is 1.000 atm, what is the partial pressure of carbon monoxide?
(Multiple Choice)
4.8/5  (43)
(43)
When a reaction system reaches equilibrium, the forward and reverse reactions stop.
(True/False)
4.8/5  (33)
(33)
Consider the following two equilibria and their respective equilibrium constants: (1) NO(g) + 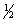 O2(g)
O2(g)  NO2(g)
(2) 2NO2(g)
NO2(g)
(2) 2NO2(g)  2NO(g) + O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
2NO(g) + O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
(Multiple Choice)
4.9/5  (42)
(42)
Methanol can be synthesized by combining carbon monoxide and hydrogen. CO(g) + 2H2(g)  CH3OH(g)
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
CH3OH(g)
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
(Multiple Choice)
4.8/5  (30)
(30)
Hydrogen bromide will dissociate into hydrogen and bromine gases. 2HBr(g)  H2(g) + Br2(g) H°rxn = 68 kJ
What effect will a temperature increase of 50°C have on this system at equilibrium?
H2(g) + Br2(g) H°rxn = 68 kJ
What effect will a temperature increase of 50°C have on this system at equilibrium?
(Multiple Choice)
4.8/5  (28)
(28)
Write the mass-action expression, Qc , for the following chemical reaction. Fe3+(aq) + 3OH-(aq)  Fe(OH)3(s)
Fe(OH)3(s)
(Multiple Choice)
4.7/5  (35)
(35)
Increasing the initial amount of the limiting reactant in a reaction will increase the value of the equilibrium constant, Kc.
(True/False)
4.7/5  (42)
(42)
The following reaction is at equilibrium in a closed container. CuSO4.5H2O(s)  CuSO4(s) + 5H2O(g)
Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
CuSO4(s) + 5H2O(g)
Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
(Multiple Choice)
4.8/5  (41)
(41)
If all the reactants and products in an equilibrium reaction are in the gas phase, then Kp = Kc.
(True/False)
4.8/5  (34)
(34)
The two equilibrium constants for the same reaction, Kc and Kp, will always equal one another when
(Multiple Choice)
4.7/5  (24)
(24)
Showing 1 - 20 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)