Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
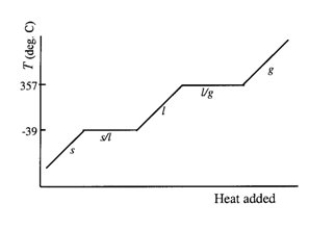
Mercury melts at -39°C and boils at 357°C. Draw a diagram of the heating curve of mercury. Label all lines and axes, and clearly indicate the melting and boiling points on your diagram.
Free
(Essay)
4.9/5  (37)
(37)
Correct Answer:
The vapor pressure of 1-butene is 1.268 atm at 273.15 K and its heat of vaporization is 22.9 kJ/mol. What is the normal boiling point of 1-butene?
Free
(Short Answer)
4.7/5  (24)
(24)
Correct Answer:
266.9 K
A sample of octane in equilibrium with its vapor in a closed 1.0-L container has a vapor pressure of 50.0 torr at 45°C. The container's volume is increased to 2.0 L at constant temperature and the liquid/vapor equilibrium is reestablished. What is the vapor pressure?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
In hydrogen iodide __________________ are the most important intermolecular forces.
(Multiple Choice)
4.8/5  (27)
(27)
Examine the following phase diagram and identify the feature represented by point A. 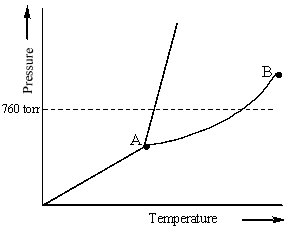
(Multiple Choice)
4.8/5  (39)
(39)
The strongest intermolecular interactions between hydrogen fluoride (HF) molecules arise from
(Multiple Choice)
4.8/5  (35)
(35)
When the electron cloud of a molecule is easily distorted, the molecule has a high _____________.
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following atoms should have the smallest polarizability?
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following pairs is arranged with the particle of higher polarizability listed first?
(Multiple Choice)
4.8/5  (37)
(37)
Use molecular orbital band diagrams to explain why metals are good conductors but semiconductors are not.
(Essay)
4.8/5  (36)
(36)
When silicon is doped with an element from group 3A(13), the device/material produced is a/an
(Multiple Choice)
4.7/5  (38)
(38)
A liquid may be made to boil at room temperature, simply by lowering the pressure.
(True/False)
5.0/5  (37)
(37)
Of the five major types of crystalline solid, which would you expect each of the following to form? (e.g., H2O: molecular)
a. Sn
b. Si
c. KCl
d. Xe
e. F2
(Essay)
5.0/5  (39)
(39)
Which of the following statements concerning a face-centered cubic unit cell and the corresponding lattice, made up of identical atoms, is incorrect?
(Multiple Choice)
4.9/5  (40)
(40)
Which one of the following substances does not exist in the indicated solid type?
(Multiple Choice)
4.9/5  (36)
(36)
A metal with a body-centered cubic lattice will have ______ atom(s) per unit cell.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is true about kinetic energy, Ek, and potential energy, Ep, when ethyl alcohol at 40°C is compared with ethyl alcohol at 20°C?
(Multiple Choice)
4.8/5  (29)
(29)
The energy of a hydrogen bond is greater than that of a typical covalent bond.
(True/False)
4.8/5  (32)
(32)
a. State the essential requirements for hydrogen bonding to be important in a compound.
b. List four properties of water which are significantly influenced by the presence of hydrogen bonding.
(Essay)
4.9/5  (32)
(32)
Showing 1 - 20 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)