Exam 1: Keys to the Study of Chemistry
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
A dictionary has the following definition for a word: "A tentative explanation that accounts for a set of facts." Which of the following words best fits that definition?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
B
Water vapor is less dense than ice because
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
E
Give an example of a physical property and a chemical property of each of the following:
a. oxygen gas
b. octane
c. copper
Free
(Essay)
4.7/5  (29)
(29)
Correct Answer:
Answers could all be the same, but some possibilities are:
a. boiling point, reaction with sodium
b. boiling point, reaction with oxygen
c. electrical conductivity, reaction with nitric acid
A student makes several measurements of the density of an unknown mineral sample. She then reports the average value of these measurements. The number of significant figures she uses in her result should be a measure of its
(Multiple Choice)
4.7/5  (38)
(38)
Briefly explain the relationship between hypothesis and experiment in the scientific method.
(Essay)
4.8/5  (34)
(34)
Which one of the following numbers contains a digit or digits which is/are not significant?
(Multiple Choice)
4.9/5  (40)
(40)
Which one of the following is a "substance" in the sense of the word as used in your textbook?
(Multiple Choice)
4.9/5  (39)
(39)
At a pressure of one billionth (10-9) of atmospheric pressure, there are about 2.7 × 1010 molecules in one cubic centimeter of a gas. How many molecules is this per cubic meter?
(Multiple Choice)
4.8/5  (38)
(38)
Use the relationship between temperatures in Celsius and Fahrenheit to calculate the temperature at which
a. the numerical value is the same on both scales.
b. the Fahrenheit number is exactly twice the Celsius number.
(Essay)
4.8/5  (39)
(39)
Select the answer that expresses the result of this calculation with the correct number of significant figures. 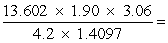
(Multiple Choice)
4.9/5  (31)
(31)
The potential energy of a car moving on a level road does not depend on its speed.
(True/False)
4.8/5  (31)
(31)
When applying the scientific method, it is important to avoid any form of hypothesis.
(True/False)
4.7/5  (30)
(30)
Talc is a mineral that has low conductivity for heat and electricity and that is not attacked by acid. It is used as talcum powder and face powder. A sample of talc weighs 35.97 g in air and 13.65 g in mineral oil (d = 1.75 g/cm3). What is the density of talc?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following abbreviations of the given SI base unit is incorrect?
(Multiple Choice)
4.9/5  (28)
(28)
The most significant contribution to modern science made by alchemists was
(Multiple Choice)
4.7/5  (28)
(28)
Showing 1 - 20 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)