Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Sucrose decomposes to fructose and glucose in acid solution. When ln [sucrose] is plotted vs. time, a straight line with slope of -0.208 hr-1 results. What is the rate law for the reaction?
Free
(Multiple Choice)
4.8/5  (45)
(45)
Correct Answer:
B
You are required to determine the energy of activation (Ea) of a reaction. Briefly describe the experimental measurements you would make and how you would obtain the activation energy from a suitable linear plot of the experimental data.
Free
(Essay)
5.0/5  (31)
(31)
Correct Answer:
Rate constants k over a suitable range of absolute temperatures T. A plot of ln k versus 1/T should be linear, and its slope is - Ea/R.
Which of the following affects the activation energy of a reaction?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
C
Which one of the following sets of units is appropriate for a second-order rate constant?
(Multiple Choice)
4.9/5  (38)
(38)
Cyclopropane is converted to propene in a first-order process. The rate constant is 5.4 × 10-2 hr-1. If the initial concentration of cyclopropane is 0.150 M, what will its concentration be after 22.0 hours?
(Multiple Choice)
4.9/5  (36)
(36)
In the gas phase at 500.°C, cyclopropane reacts to form propene in a first-order reaction. The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L) plotted versus time. 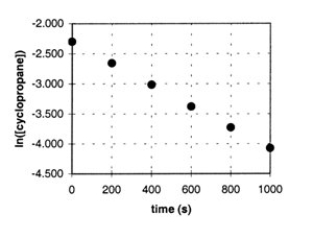 a. Explain how this plot confirms that the reaction is first order.
b. Calculate the first-order rate constant, k.
c. Determine the initial concentration of
cyclopropane in this experiment.
a. Explain how this plot confirms that the reaction is first order.
b. Calculate the first-order rate constant, k.
c. Determine the initial concentration of
cyclopropane in this experiment.
(Essay)
4.8/5  (36)
(36)
For the reaction A(g) + 2B(g) 2C(g) + 2D(g)
The following data were collected at constant temperature. Determine the correct rate law for this reaction. 
(Multiple Choice)
4.8/5  (30)
(30)
A reaction has an activation energy of 195.0 kJ/mol. When the temperature is increased from 200.°C to 220.°C, the rate constant will increase by a factor of
(Multiple Choice)
4.8/5  (35)
(35)
A reaction intermediate is a species corresponding to a local energy maximum on a reaction energy diagram.
(True/False)
4.9/5  (32)
(32)
The greater the energy of activation, Ea, the faster will be the reaction.
(True/False)
4.8/5  (23)
(23)
A rate constant obeys the Arrhenius equation, the factor A being 2.2 × 1013 s-1 and the activation energy being 150. kJ mol-1. What is the value of the rate constant at 227°C, in s-1?
(Multiple Choice)
4.8/5  (28)
(28)
The half-life of a first-order reaction does not depend on the initial concentration of reactant.
(True/False)
4.9/5  (31)
(31)
The decomposition of hydrogen peroxide is a first-order process with a rate constant of 1.06 × 10-3 min-1. How long will it take for the concentration of H2O2 to drop from 0.0200 M to 0.0120 M?
(Multiple Choice)
4.9/5  (33)
(33)
Carbon-14 is a radioactive isotope which decays with a half-life of 5730 years. What is the first-order rate constant for its decay, in units of years-1?
(Multiple Choice)
4.9/5  (42)
(42)
The rate law for the reaction 3A 2B is rate = k[A] with a rate constant of 0.0447 hr-1. What is the half-life of the reaction?
(Multiple Choice)
4.8/5  (34)
(34)
You are studying the rate of the reaction 2A B and have obtained measurements of the concentration of A at times t = 100, 200, 300, ......, 1000 seconds from the start of the reaction. Carefully describe how you would plot a graph and use it to
a. prove that the reaction is second-order with respect to A.
b. determine the second-order rate constant k.
(Essay)
4.8/5  (39)
(39)
Is a bimolecular reaction necessarily second-order? Is a second-order reaction necessarily bimolecular? Answer, with explanations and clarifications.
(Essay)
4.9/5  (29)
(29)
Which of the following sets of units could be appropriate for a zero-order rate constant?
(Multiple Choice)
4.9/5  (37)
(37)
The active ingredient in an over the counter pain killer analgesic decomposes with a rate constant, k = 9.05 × 10-4 day-1. How many days does it take for 15% of the original ingredient to decompose?
(Multiple Choice)
4.9/5  (45)
(45)
Showing 1 - 20 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)