Exam 9: Models of Chemical Bonding
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
The more C-O and O-H bonds there are in a substance, the greater will be the amount of heat released when a fixed mass of the substance is burned.
Free
(True/False)
4.9/5  (37)
(37)
Correct Answer:
False
A Born-Haber cycle applied to the formation reaction of an ionic solid
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
E
In not more than three sentences, describe the electron arrangement responsible for bonding in solid SrCl2.
Free
(Essay)
4.9/5  (37)
(37)
Correct Answer:
This is an example of ionic bonding in which Sr, from group 2A (2) will be present as Sr2+ ions, while Cl, from group 7A (7) will be present as Cl- ions. The cations and anions will be arranged in a crystalline lattice so that nearest neighbors will be ions of opposite charge, thus achieving a net coulombic attraction. There are no molecules present; each ion is equally attracted to all its nearest neighbors.
Quartz (SiO2) is a solid with a melting point of 1550 °C. The bonding in quartz is best described as
(Multiple Choice)
4.8/5  (32)
(32)
A single covalent bond consists of a single delocalized electron pair.
(True/False)
4.9/5  (29)
(29)
Select the compound with the lowest (i.e., least negative) lattice energy.
(Multiple Choice)
4.7/5  (35)
(35)
Give a clear and concise definition of the term "electronegativity"; i.e., what does it measure?
(Essay)
4.9/5  (36)
(36)
As a measure of the strength of metallic bonding, the boiling point of a metal is a better indicator than its melting point.
(True/False)
4.7/5  (36)
(36)
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given. 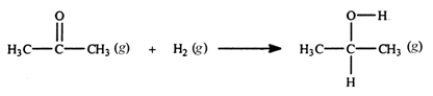

(Multiple Choice)
4.9/5  (33)
(33)
Which of the following compounds displays the greatest ionic character in its bonds?
(Multiple Choice)
4.8/5  (31)
(31)
When one mole of each of the following liquids is burned, which will produce the most heat energy?
(Multiple Choice)
4.8/5  (39)
(39)
The stronger the bonds in a fuel, the more energy it will yield.
(True/False)
4.9/5  (34)
(34)
Electronegativities on Pauling's scale are calculated from ionization energies and electron affinities.
(True/False)
4.8/5  (33)
(33)
The lattice energy of MgCl2 is the energy change for which one of the following processes?
(Multiple Choice)
4.8/5  (28)
(28)
Most of the copper sold in major metal markets is highly purified, typically to 99.99%. Why is this?
(Essay)
4.8/5  (41)
(41)
Select the correct formula for a compound formed from calcium and chlorine.
(Multiple Choice)
4.9/5  (26)
(26)
Using appropriate, real examples to illustrate your answer, describe the correlation between bond energy and bond length for a series of single bonds.
(Essay)
4.8/5  (34)
(34)
A hypothetical ionic substance will not form merely because it has a high lattice energy. Explain why, using energy-based arguments.
(Essay)
4.9/5  (39)
(39)
Showing 1 - 20 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)