Exam 16: Solubility and Complex Ion Equilibria
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
Barium carbonate has a measured solubility of 4.03 10-5 at 25°C.Determine the Ksp.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following compounds has the lowest solubility in mol/L in water at 25°C?
(Multiple Choice)
4.7/5  (38)
(38)
Find the solubility (in mol/L)of lead(II)chloride,PbCl2,at 25°C.Ksp = 1.59 10-5.
(Multiple Choice)
4.8/5  (38)
(38)
The solubility in mol/L of M(OH)2 in 0.053 M KOH is 1.0 10-5 mol/L.What is the Ksp for M(OH)2?
(Multiple Choice)
4.7/5  (30)
(30)
What is the concentration of Ni2+(aq)ion in a 0.045 M Ni(NO3)2 solution that is also 1.0 M NH3? [Kf for Ni(NH3)62+ = 5.5 108]
(Multiple Choice)
4.9/5  (44)
(44)
A 300.0-mL saturated solution of copper(II)peroidate,Cu(IO4)2,contains 0.30 grams of dissolved salt.Determine the Ksp.
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the concentration of the silver ion in a saturated solution of silver chloride,AgCl (Ksp = 1.57 10-10).
(Multiple Choice)
4.8/5  (40)
(40)
The solubility in mol/L of Ag2CrO4 is 1.8 10-4 M.Calculate the Ksp for this compound.
(Multiple Choice)
4.7/5  (35)
(35)
The Kf for the complex ion Ag(NH3)2+ is 1.7 107.The Ksp for AgCl is 1.6 10-10.Calculate the molar solubility of AgCl in 1.0 M NH3.
(Multiple Choice)
4.9/5  (40)
(40)
In the classic scheme for qualitative analysis,the cations of Analytical Group IV are precipitated as phosphates or carbonates.Analytical Group IV consists of
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following solid salts is more soluble in 1.0 M H+ than in pure water?
(Multiple Choice)
4.9/5  (48)
(48)
The cation M2+ reacts with NH3 to form a series of complex ions as follows: M2+ + NH3
 M(NH3)2+
K1 = 102
M(NH3)2+ + NH3
M(NH3)2+
K1 = 102
M(NH3)2+ + NH3
 M(NH3)22+
K2 = 103
M(NH3)22+ + NH3
M(NH3)22+
K2 = 103
M(NH3)22+ + NH3
 M(NH3)32+
K3 = 102
A 1.0 10-3 mol sample of M(NO3)2 is added to 1.0 L of 15.0 M NH3 (Kb = 1.8 10-5).Choose the dominant species in this solution.
M(NH3)32+
K3 = 102
A 1.0 10-3 mol sample of M(NO3)2 is added to 1.0 L of 15.0 M NH3 (Kb = 1.8 10-5).Choose the dominant species in this solution.
(Multiple Choice)
4.7/5  (29)
(29)
The solubility of an unknown salt,M2Z,in a 0.0768 M CaZ solution is 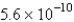 mol/L.Calculate the Ksp for M2Z.
mol/L.Calculate the Ksp for M2Z.
(Multiple Choice)
5.0/5  (37)
(37)
Calculate the molar concentration of uncomplexed Zn2+ in a solution that contains 0.20 mole of Zn(NH3)42+ per liter and 0.0116 M NH3 at equilibrium.The overall Kf for Zn(NH3)42+ is 3.8 109.
(Multiple Choice)
4.8/5  (41)
(41)
Silver acetate,AgC2H3O2, is a sparingly soluble salt with Ksp = 1.9 10-3.Consider a saturated solution in equilibrium with the solid salt.Compare the effects on the solubility of adding to the solution either the acid HNO3 or the base NH3.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following solid salts should be more soluble in 1.0 M NH3 than in water?
(Multiple Choice)
4.9/5  (34)
(34)
When a mixture containing cations of Analytical Groups I-III is treated with H2S in acidic solution,which cations are expected to precipitate?
(Multiple Choice)
4.8/5  (46)
(46)
The solubility of silver phosphate,Ag3PO4,at 25°C is 1.55 10-5 mol/L.Determine the concentration of the Ag+ ion in a saturated solution.
(Multiple Choice)
4.8/5  (45)
(45)
How many moles of CaF2 will dissolve in 3.0 liters of 0.089 M NaF solution? (Ksp for CaF2 = 4.0 10-11)
(Multiple Choice)
4.8/5  (27)
(27)
The following questions refer to the following system: 3.5 102 mL of 3.2 M Pb(NO3)2 and 2.0 102 mL of 0.020 M NaCl are added together.Ksp for the lead chloride is 1.6 10-5.
-Determine the ion product.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)