Exam 16: Solubility and Complex Ion Equilibria
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
In a solution prepared by adding excess PbI2 (Ksp = 1.44 10-8)to water,the [I-] at equilibrium is:
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following solid salts is more soluble in 1.0 M H+ than in pure water?
(Multiple Choice)
4.9/5  (36)
(36)
An unknown salt,M3Z,has a Ksp of 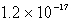 .Calculate the solubility in mol/L of M3Z.
.Calculate the solubility in mol/L of M3Z.
(Multiple Choice)
4.9/5  (36)
(36)
Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3.Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3)2+:
Ag+ + NH3
 AgNH3+
K1 = 2.1 103
AgNH3+ + NH3
AgNH3+
K1 = 2.1 103
AgNH3+ + NH3
 Ag(NH3)2+
K2 = 8.2 103
-The concentration of Ag+ at equilibrium is:
Ag(NH3)2+
K2 = 8.2 103
-The concentration of Ag+ at equilibrium is:
(Multiple Choice)
4.8/5  (40)
(40)
You have a solution consisting of 0.10 M Cl- and 0.10 M CrO42-.You add 0.10 M silver nitrate dropwise to this solution.Given that the Ksp for Ag2CrO4 is 9.0 10-12,and that for AgCl is 1.6 10-10,which of the following will precipitate first?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the concentration of Al3+ in a saturated aqueous solution of Al(OH)3 (Ksp = 2.2 10-32).
(Multiple Choice)
4.7/5  (30)
(30)
The correct mathematical expression for finding the molar solubility (s)of Sn(OH)2 is:
(Multiple Choice)
4.8/5  (40)
(40)
Solubility Products (Ksp) 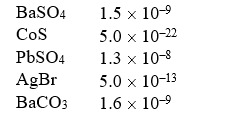 Which of the following compounds is the most soluble (in moles/liter)?
Which of the following compounds is the most soluble (in moles/liter)?
(Multiple Choice)
4.9/5  (40)
(40)
The Ksp of PbSO4 is 1.3 10-8.Calculate the solubility (in mol/L)of PbSO4 in a 0.0037 M solution of Na2SO4.
(Multiple Choice)
4.9/5  (30)
(30)
Sodium chloride is added slowly to a solution that is 0.010 M in Cu+,Ag+,and Au+.The Ksp values for the chloride salts are 1.9 10-7,1.6 10-10,and 2.0 10-13,respectively.Which compound will precipitate first?
(Multiple Choice)
4.8/5  (38)
(38)
The molar solubility of BaCO3 (Ksp = 1.6 10-9)in 0.10 M BaCl2 solution is:
(Multiple Choice)
4.8/5  (32)
(32)
The solubility of an unknown salt,M3Z2, at 25°C is 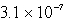 mol/L.What is the Ksp for M3Z2 at 25°C?
mol/L.What is the Ksp for M3Z2 at 25°C?
(Multiple Choice)
4.8/5  (37)
(37)
The solubility of AgCl in water is _____ the solubility of AgCl in strong acid at the same temperature.
(Multiple Choice)
4.9/5  (35)
(35)
The solubility of Cd(OH)2 in water is 1.67 10-5 mol/L.The Ksp value for Cd(OH)2 is
(Multiple Choice)
4.9/5  (34)
(34)
A 100.-mL sample of solution contains 10.0 mmol of Ca2+ ion.How many mmol of solid Na2SO4 must be added in order to cause precipitation of 99.9% of the calcium as CaSO4? The Ksp of CaSO4 is 6.1 10-5.Assume the volume remains constant.
(Multiple Choice)
4.8/5  (44)
(44)
The concentration of Mg2+ in seawater is 0.052 M.At what pH will 99% of the Mg2+ be precipitated as the hydroxide? [Ksp for Mg(OH)2 = 8.9 10-12]
(Multiple Choice)
4.9/5  (27)
(27)
A 50.0-mL sample of 0.100 M Ca(NO3)2 is mixed with 50.00 mL of 0.200 M NaF.When the system has come to equilibrium,which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 10-11. Moles Solid CaF2
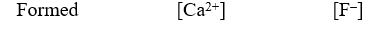
(Multiple Choice)
4.7/5  (32)
(32)
The solubility of an unknown salt,MZ2, at 25°C is 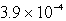 mol/L.What is the Ksp for MZ2 at 25°C?
mol/L.What is the Ksp for MZ2 at 25°C?
(Multiple Choice)
4.8/5  (35)
(35)
The Ksp of an unknown salt,MZ2,is 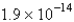 .Calculate the solubility (in mol/L)of MZ2 in a 0.0230 M solution of CaZ2.
.Calculate the solubility (in mol/L)of MZ2 in a 0.0230 M solution of CaZ2.
(Multiple Choice)
4.9/5  (47)
(47)
Which of the following compounds has the lowest solubility in mol/L in water?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 41 - 60 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)