Exam 16: Solubility and Complex Ion Equilibria
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
You have two salts,AgX and AgY,with very similar Ksp values.You know that Ka for HX is much greater than Ka for HY.Which salt is more soluble in acidic solution?
(Multiple Choice)
4.9/5  (35)
(35)
What is the molar solubility of lead(II)chromate in 0.055 M Na2S2O3? For PbCrO4,Ksp = 2.0 10-16;for Pb(S2O3)34-,Kf = 2.2 106.
(Multiple Choice)
4.9/5  (47)
(47)
The Ksp for BaF2 is 2.4 10-5.When 10 mL of 0.01 M NaF is mixed with 10 mL of 0.01 M BaNO3,will a precipitate form?
(Multiple Choice)
4.9/5  (34)
(34)
The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO3)2 are mixed with 1.0 L of 1.0 M Na2C2O4.The oxalate ion,C2O4,acts as a ligand to form a complex ion with the Mn2+ ion with a coordination number of two.
Mn2+ + C2O42-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -Find the equilibrium concentration of Mn(C<sub>2</sub>O<sub>4</sub>)in the system.](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d99_93a6_21fcac416c0b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -Find the equilibrium concentration of Mn(C<sub>2</sub>O<sub>4</sub>)in the system.](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d9a_93a6_fd88b5379204_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4 + C2O42-
K2 = 1.26 10-2
-Find the equilibrium concentration of Mn(C2O4)in the system.
MnC2O4 + C2O42-
K2 = 1.26 10-2
-Find the equilibrium concentration of Mn(C2O4)in the system.
(Multiple Choice)
4.7/5  (41)
(41)
The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO3)2 are mixed with 1.0 L of 1.0 M Na2C2O4.The oxalate ion,C2O4,acts as a ligand to form a complex ion with the Mn2+ ion with a coordination number of two.
Mn2+ + C2O42-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -Find the equilibrium concentration of the [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> ion.](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d99_93a6_21fcac416c0b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -Find the equilibrium concentration of the [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> ion.](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d9a_93a6_fd88b5379204_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4 + C2O42-
K2 = 1.26 10-2
-Find the equilibrium concentration of the [Mn(C2O4)2]2- ion.
MnC2O4 + C2O42-
K2 = 1.26 10-2
-Find the equilibrium concentration of the [Mn(C2O4)2]2- ion.
(Multiple Choice)
4.8/5  (36)
(36)
The Ksp for PbF2 is 4.0 10-8.If a 0.040 M NaF solution is saturated with PbF2,what is the [Pb2+] in the solution?
(Multiple Choice)
4.9/5  (37)
(37)
What is the best way to ensure complete precipitation of SnS from a saturated H2S solution?
(Multiple Choice)
4.9/5  (42)
(42)
How many moles of Fe(OH)2 [Ksp = 1.8 10-15] will dissolve in 1.0 liter of water buffered at pH = 10.37?
(Multiple Choice)
4.8/5  (31)
(31)
If 30 mL of 5.0 10-4 M Ca(NO3)2 are added to 70 mL of 2.0 10-4 M NaF,will a precipitate occur? (Ksp of CaF2 = 4.0 10-11)
(Multiple Choice)
4.7/5  (36)
(36)
The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO3)2 are mixed with 1.0 L of 1.0 M Na2C2O4.The oxalate ion,C2O4,acts as a ligand to form a complex ion with the Mn2+ ion with a coordination number of two.
Mn2+ + C2O42-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -Find the equilibrium concentration of the Mn<sup>2+</sup> ion.](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d99_93a6_21fcac416c0b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -Find the equilibrium concentration of the Mn<sup>2+</sup> ion.](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d9a_93a6_fd88b5379204_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4 + C2O42-
K2 = 1.26 10-2
-Find the equilibrium concentration of the Mn2+ ion.
MnC2O4 + C2O42-
K2 = 1.26 10-2
-Find the equilibrium concentration of the Mn2+ ion.
(Multiple Choice)
4.8/5  (44)
(44)
Calculate the solubility of Cu(OH)2 in a solution buffered at pH = 7.59.(Ksp = 1.6 10-19)
(Multiple Choice)
4.9/5  (39)
(39)
The 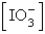 in a saturated solution of
in a saturated solution of 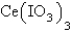 is 5.93 10-3 M.Calculate the Ksp for
is 5.93 10-3 M.Calculate the Ksp for 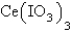 .
.
(Multiple Choice)
4.9/5  (32)
(32)
A 0.012-mol sample of Na2SO4 is added to 400 mL of each of two solutions.One solution contains 1.5 10-3 M BaCl2;the other contains 1.5 10-3 M CaCl2.Given that Ksp for BaSO4 = 1.5 10-9 and Ksp for CaSO4 = 6.1 10-5:
(Multiple Choice)
4.8/5  (34)
(34)
It is observed that 7.50 mmol of BaF2 will dissolve in 1.0 L of water.Use these data to calculate the value of Ksp for barium fluoride.
(Multiple Choice)
4.7/5  (32)
(32)
In the qualitative analysis scheme for metal ions,how are the Analytical Group III cations separated from the cations of Analytical Groups IV and V?
(Multiple Choice)
4.8/5  (39)
(39)
What is the maximum concentration of carbonate ions that will precipitate BaCO3 but not MgCO3 from a solution that is 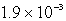 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.
(Multiple Choice)
4.8/5  (33)
(33)
The concentration of OH- in a saturated solution of Mg(OH)2 is 3.63 10-4 M.The Ksp of Mg(OH)2 is
(Multiple Choice)
4.9/5  (35)
(35)
A solution is 0.010 M in each of Pb(NO3)2,Mn(NO3)2,and Zn(NO3)2.Solid NaOH is added until the pH of the solution is 8.50.Which of the following statements is true?

(Multiple Choice)
4.9/5  (32)
(32)
Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3.Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3)2+:
Ag+ + NH3
 AgNH3+
K1 = 2.1 103
AgNH3+ + NH3
AgNH3+
K1 = 2.1 103
AgNH3+ + NH3
 Ag(NH3)2+
K2 = 8.2 103
-The concentration of Ag(NH3)2+ at equilibrium is:
Ag(NH3)2+
K2 = 8.2 103
-The concentration of Ag(NH3)2+ at equilibrium is:
(Multiple Choice)
4.8/5  (33)
(33)
Showing 61 - 80 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)