Exam 11: Solutions: Properties and Behavior
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Describe how the molar mass of a compound can be determined from data obtained in two experiments: (1) measurements of the freezing point, and (2) measurements of the boiling point of a solution of the compound.
(Essay)
4.8/5  (42)
(42)
Identify the following statement as true or false and choose the correct explanation. "For solutions with the same reverse osmotic pressure at the same temperature, the molarity of a sodium chloride solution will always be less than the molarity of a calcium chloride solution."
(Multiple Choice)
4.9/5  (38)
(38)
Ion interaction energies are determined by Coulomb's law: 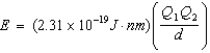 Which of the following compounds has an interaction energy 3.83 1018 J?
Which of the following compounds has an interaction energy 3.83 1018 J?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following compounds has the largest melting point?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following solutions, assuming equal volumes and a nonvolatile solute, would have the lowest boiling point?
(Multiple Choice)
4.8/5  (37)
(37)
A solution contains 6.50 mol water, 0.300 mol sucrose, and 0.200 mol glucose. The solutes are nonvolatile. What is the vapor pressure of the solution at 35C given that the vapor pressure of water is 42.2 torr?
(Multiple Choice)
4.8/5  (31)
(31)
Seawater can be characterized by the following average values. What is the molal concentration of ions in seawater? Explain how you arrived at your answer.
density 1.022 g/mL
total mass of ions 35.17 g/kg
concentration of ions 1.15 M
(Essay)
4.7/5  (42)
(42)
Which of the following must be true if the dissolution of an ionic solid in water is exothermic?
(Multiple Choice)
4.8/5  (37)
(37)
Cholesterol (386.7 g/mol) is a nonelectrolyte and a critical component of the cell membranes of animal cells. What would be the change in boiling point of a solution containing 1.23 g of cholesterol and 105 g of chloroform (Kb 3.63C/m)?
(Multiple Choice)
4.7/5  (35)
(35)
Ethylene glycol is used commonly as an antifreeze in automobile radiators. Automobile temperatures in the U.S. are commonly measured in degrees Fahrenheit. What is the freezing point in F of a 50:50 mixture of ethylene glycol and water by volume? (C2H6O2, 62.07 g/mol, 1.1132 g/mL, Kf 3.35F/m).
(Short Answer)
4.9/5  (44)
(44)
What is the boiling point of a 3.25 m KCl solution (Kb 0.52C/m for water)?
(Multiple Choice)
4.8/5  (31)
(31)
Which one of the ionic compounds below would you expect to have the smallest (least negative) lattice energy?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following will require the greatest energy input to separate the ions?
(Multiple Choice)
4.8/5  (33)
(33)
A solution is prepared by mixing 75 g of benzene (C6H6) with 25 g of toluene (C7H8). Use the following data to determine the vapor pressure of this solution at 20C. Substance
Vapor Pressure at 20C (torr)
Benzene
75
Toluene
22
(Multiple Choice)
4.8/5  (27)
(27)
What is the molality of a solution produced by dissolving 14.40 g of LiCl (42.39 g/mol) in water to make 0.104 L of solution with a density of 1.102 g/mL?
(Multiple Choice)
4.9/5  (35)
(35)
What is the molarity of a sucrose (C12H22O11) solution that produces an osmotic pressure of 2.65 atm at 25C?
(Multiple Choice)
4.9/5  (42)
(42)
Predict the relationship between the lattice energies (U) of KBr and CaO.
(Multiple Choice)
4.7/5  (34)
(34)
Which statement regarding osmotic pressure is not correct? Osmotic pressure ________
(Multiple Choice)
4.9/5  (34)
(34)
Which listing of ionic compounds is in order of increasing melting point?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 61 - 80 of 150
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)