Exam 11: Solutions: Properties and Behavior
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Coulomb's law states that the interaction energy between ions depends ________
(Multiple Choice)
4.8/5  (47)
(47)
Rank the following ionic compounds based on their expected solubility. Provide a brief rationale for your rankings.
MgF2, KCl, and Al2S3
(Essay)
4.9/5  (36)
(36)
A solution is prepared by adding 0.250 mol napthalene, which is not volatile, to 2.25 mol diethyl ether. What is the vapor pressure of this solution at 25C given that the vapor pressure of pure diethyl ether is 532 torr?
(Short Answer)
4.8/5  (33)
(33)
Which graph best describes how the vapor pressure of a substance varies with temperature according to the Clausius-Clapeyron equation? ln(P) is plotted on the y-axis, and 1/T is plotted on the x-axis. The origin (0, 0) is not necessarily located where the axes cross. 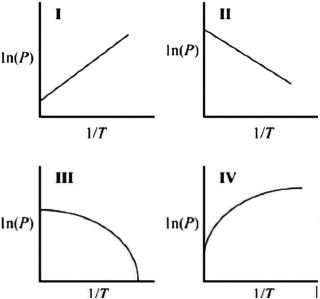
(Multiple Choice)
4.9/5  (41)
(41)
What is the molarity of a 0.923 m NaF (41.99 g/mol) solution prepared using 234 g of water? The density of the solution is 1.13 g/mL.
(Multiple Choice)
4.8/5  (35)
(35)
In the following Born-Haber cycle for the formation of sodium chloride from its elements, which step (A-E) corresponds to the lattice energy for NaCl? 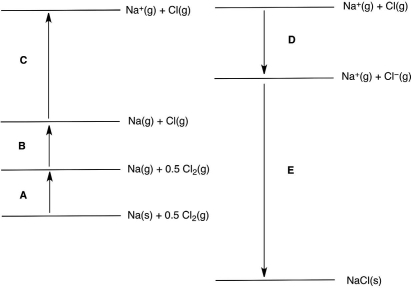
(Multiple Choice)
4.9/5  (43)
(43)
A newspaper article suggested using a fertilizer such as ammonium sulfate or ammonium nitrate to lower the melting point of ice on sidewalks because many of the deicing salts can damage lawns and sidewalks. Which of the following compounds would give the largest freezing point depression when 100 g of the compound are dissolved in 1 kg of solvent?
(Multiple Choice)
4.7/5  (30)
(30)
In the following Born-Haber cycle for the formation of sodium chloride from its elements, which step (A-E) corresponds to the electron affinity of one of the intermediate species? 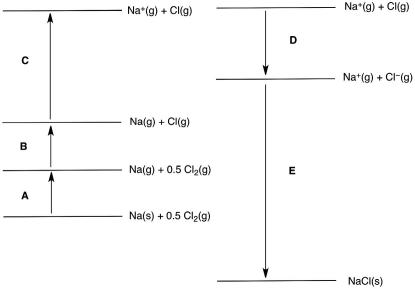
(Multiple Choice)
4.8/5  (43)
(43)
A solution is prepared by adding 1.50 mol glucose, which is not volatile, to 3.50 mol diethyl ether. What is the vapor pressure of this solution at 25C given that the vapor pressure of pure diethyl ether is 23.8 torr?
(Multiple Choice)
4.9/5  (33)
(33)
What would be the boiling point of a 0.52 m solution of KBr in water (Kb 0.52C)?
(Multiple Choice)
4.8/5  (37)
(37)
Calculate the lattice energy of magnesium chloride from the following data: First ionization energy of Mg: 738 kJ/mol
Second ionization energy of Mg: 1,451 kJ/mol
Electron affinity of Cl: 349 kJ/mol
Energy to vaporize Mg: 147.1 kJ/mol
Cl2 bond energy: 243 kJ/mol
Energy change for the reaction: 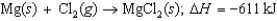
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following ionic compounds would you predict to have the lowest solubility in water?
(Multiple Choice)
4.9/5  (39)
(39)
What would be the freezing point of a 1 m solution of HCl in water (Kf 1.86C)?
(Multiple Choice)
4.9/5  (37)
(37)
Click, Clack, and Jim have identical icy driveways. Click buys 10 lb of NaCl, Clack buys 10 lb of CaCl2, and Jim buys 10 lb of MgCl2. They spread these salts on their driveways. Which driveway will deice more effectively? (Assume that ideal dissociation of the salts occurs, and that colligative properties are the only consideration.)
(Multiple Choice)
4.7/5  (39)
(39)
Which one of the ionic compounds below would you expect to have the largest (most negative) lattice energy?
(Multiple Choice)
4.8/5  (33)
(33)
A solution of 5.00 g of sodium chloride in 1.00 kg of water has a freezing point of 0.299C. What is the actual van 't Hoff factor for this salt at this concentration compared to the ideal one of 2? Kf (water) 1.86C/m
(Multiple Choice)
4.8/5  (41)
(41)
You are working as an intern in a biochemistry lab. Your advisor just isolated an enzyme and asks you to determine its molar mass using osmosis. You dissolve 1.0 g of the enzyme in water to make 250 mL of solution. You measure the osmotic pressure and find it to be 3.5 torr at 298 K. You report that the molar mass is ________ g/mol.
(Multiple Choice)
4.8/5  (33)
(33)
Determine the molal concentration of a sugar solution in water that has a freezing point of 2.1C. Kf 1.86C/m for water.
(Multiple Choice)
5.0/5  (35)
(35)
Which statement about how the vapor pressure (P) of a liquid depends on temperature is correct?
(Multiple Choice)
4.8/5  (36)
(36)
Describe how the data obtained by a measurement of osmotic pressure can be used to determine the molar mass of a compound.
(Essay)
4.9/5  (33)
(33)
Showing 121 - 140 of 150
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)