Exam 11: Solutions: Properties and Behavior
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The molal concentration of ions in seawater is 1.17 m, and the molar concentration is 1.15 M. What is the minimum pressure that must be applied to produce pure water from seawater through reverse osmosis at 25C?
(Short Answer)
4.8/5  (40)
(40)
At 25C, the vapor pressure of pure water is 25.756 mm Hg. What is the vapor pressure of water in a 0.500 m solution of sodium chloride?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following will have the largest cation-anion attraction?
(Multiple Choice)
4.7/5  (35)
(35)
Coulomb's law states that the energy of attraction between ions depends ________
(Multiple Choice)
4.7/5  (35)
(35)
Indicate which aqueous solution has the slowest evaporation rate.
(Multiple Choice)
4.8/5  (29)
(29)
Write out the five steps of the Born-Haber cycle for formation of solid sodium chloride from its elements. Label what energy each step corresponds to. Also, note which steps are exo- or endothermic.
(Essay)
4.8/5  (31)
(31)
In the following Born-Haber cycle for the formation of sodium chloride from its elements, which step (A-E) corresponds to a molar heat of sublimation for one of the reactants? 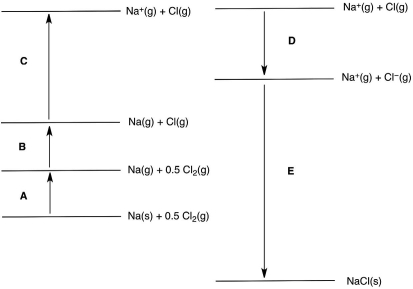
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following compounds has the lowest melting point?
(Multiple Choice)
4.8/5  (41)
(41)
In the following Born-Haber cycle for the formation of sodium chloride from its elements, which step (A-E) corresponds to the ionization energy of one of the reactants? 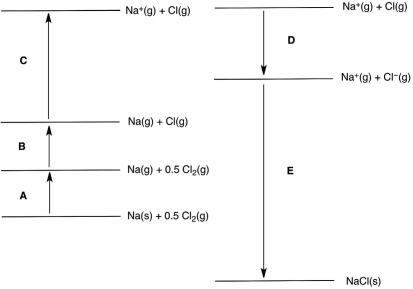
(Multiple Choice)
4.8/5  (38)
(38)
Suppose 100.0 mL of a 2.50 mM NaCl solution were mixed with 100.0 mL of a 3.40 mM MgCl2 solution at 25C. Assuming the volumes are additive and using the ideal van 't Hoff factors, what would be the osmotic pressure of the resulting solution?
(Short Answer)
4.8/5  (31)
(31)
Identify the following statement as true or false and choose the correct explanation. "For solutions with the same molarity at the same temperature, the pressure needed for reverse osmosis of a sodium chloride solution will always be less than the pressure needed for a calcium chloride solution."
(Multiple Choice)
4.8/5  (36)
(36)
In the process of dialysis, a special semipermeable membrane allows both small molecules and water to pass through, but not large protein molecules. These membranes are used to separate these small molecules and ions from much larger proteins. If a mixture of proteins and small molecules were separated from pure water by a dialysis membrane as shown in the figure, which way would the molecules flow? 
(Multiple Choice)
4.8/5  (36)
(36)
What mass of a 1.23 m sodium carbonate (105.99 g/mol) solution will contain 0.23 mol of solute?
(Multiple Choice)
4.9/5  (34)
(34)
The freezing point of a 0.060 m solution of magnesium sulfate (MgSO4) is 0.18C. What is the value of the van 't Hoff factor for this nonideal solution? Kf for water is 1.86C/m.
(Multiple Choice)
4.7/5  (41)
(41)
A petroleum company separates benzene (78 g/mol) and n-octane (114 g/mol) by distillation. In one run, the starting solution was 15% benzene and 85% n-octane by mass at 25C. In the liquid, the mole ratio of benzene to n-octane was ________, and in the vapor it was ________. The vapor pressures for benzene and n-octane at this temperature are 11 torr and 95 torr, respectively.
(Multiple Choice)
4.8/5  (32)
(32)
Ion interaction energies are determined by Coulomb's law: 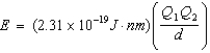 Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following solutions, assuming equal volumes and a nonvolatile solute, would have the highest boiling point? Provide rationale for your choice. 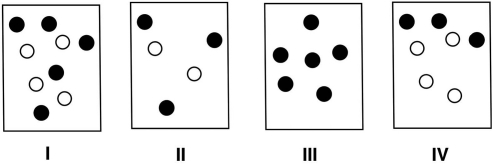
(Essay)
4.9/5  (39)
(39)
Indicate which aqueous solution has the lowest vapor pressure.
(Multiple Choice)
4.8/5  (31)
(31)
Showing 81 - 100 of 150
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)