Exam 11: Solutions: Properties and Behavior
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The normal temperature range of the liquid phase of pure water is 0C to 100C. Which of the following solutions will have the largest temperature range for the liquid state?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following will require the greatest energy input to separate the ions?
(Multiple Choice)
4.8/5  (36)
(36)
The vapor pressure of an aqueous solution is found to be 24.9 mm Hg at 25C. What is the mole fraction of solute in this solution? The vapor pressure of water is 25.756 mm Hg at 25C.
(Multiple Choice)
4.9/5  (31)
(31)
Which has the higher vapor pressure at a given temperature, pure water or salty seawater? Explain.
(Essay)
4.7/5  (40)
(40)
Determine the energy change for the reaction 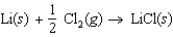 from the following data: Lattice energy of LiCl 861 kJ/mol
Energy to vaporize Li 159 kJ/mol
Ionization energy of Li 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: 349 kJ/mol
from the following data: Lattice energy of LiCl 861 kJ/mol
Energy to vaporize Li 159 kJ/mol
Ionization energy of Li 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: 349 kJ/mol
(Multiple Choice)
4.9/5  (34)
(34)
Write out the steps of the Born-Haber cycle for formation of solid magnesium iodide from its elements. Label what energy each step corresponds to. Also, describe how you would calculate the lattice energy for the compound using the diagram.
(Essay)
5.0/5  (37)
(37)
Which graph best describes how the vapor pressure of a solution varies according to Raoult's law as a nonvolatile solute is added to a liquid? The vapor pressure of the solution is plotted on the y-axis, and the mole fraction of solvent is plotted on the x-axis. The origin (0, 0) is not necessarily located where the axes cross. 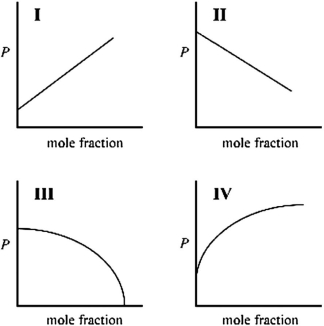
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following will have the smallest lattice energy?
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the molality of a solution containing 0.355 mol sodium hydrogen carbonate (baking soda) and 245 g of water.
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following would be most effective in melting ice on a sidewalk?
(Multiple Choice)
4.7/5  (42)
(42)
A 15.0 mg sample of a protein was dissolved in water to produce 5.00 mL of solution at 25.1C. The osmotic pressure of this solution was measured and found to be 6.50 torr. What is the molar mass of this protein?
(Multiple Choice)
4.8/5  (42)
(42)
The interaction energy of LiF is 1.14 1018 J. What is the distance between the Li and F ions?
(Multiple Choice)
4.9/5  (44)
(44)
Given the same number of moles of each of the following substances, which one would be most effective in melting ice on a sidewalk?
(Multiple Choice)
4.9/5  (48)
(48)
Which graph best describes how the vapor pressure of a solution varies according to Raoult's law as a nonvolatile solute is added to a liquid? The vapor pressure of the solution is plotted on the y-axis, and the mole fraction of solute is plotted on the x-axis. The origin (0, 0) is not necessarily located where the axes cross. 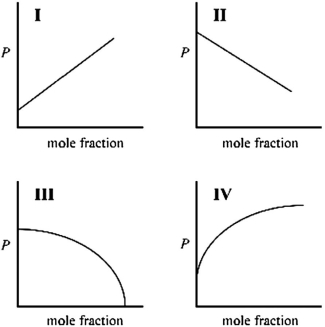
(Multiple Choice)
4.9/5  (49)
(49)
Indicate which aqueous solution has the highest vapor pressure.
(Multiple Choice)
4.8/5  (30)
(30)
Caryophyllene, a nonelectrolyte, is one of the compounds responsible for the flavor of cloves. A 207 mg sample of caryophyllene was dissolved in 1.00 g of chloroform (Kb 3.63C/m), increasing the boiling point of chloroform by 3.68C. What is the molar mass of caryophyllene?
(Multiple Choice)
5.0/5  (41)
(41)
Calculate the minimum pressure that must be applied to achieve reverse osmosis of 0.504 M NaCl at 22C.
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the molality of a solution containing 0.755 mol glucose and 1.75 kg of water.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following requires the smallest energy to separate the ions?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 41 - 60 of 150
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)