Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The temperature at point b in the phase diagram below is the ________ 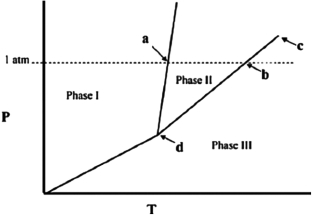
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following substances is a solid at 25C and 1 atm?
(Multiple Choice)
4.8/5  (45)
(45)
Which is the dominant interaction between carbon tetrafluoride molecules, CF4?
(Multiple Choice)
4.8/5  (36)
(36)
The smell of fresh-cut pine is due in part to a cyclic alkene called pinene. A graph of the natural logarithm of the vapor pressure of pinene vs. 1/temperature produces a straight line with a slope of  4,936.37 K. What is the enthalpy of vaporization of pinene?
4,936.37 K. What is the enthalpy of vaporization of pinene?
(Multiple Choice)
4.7/5  (44)
(44)
Describe the similarity and the difference between sublimation and evaporation.
(Essay)
4.9/5  (40)
(40)
Identify all of the intermolecular forces present in each of the following molecules.
(CH3)2CO, H2O, and N2
(Essay)
4.8/5  (38)
(38)
Which of the following compounds would be most soluble in carbon tetrachloride, CCl4?
(Multiple Choice)
4.9/5  (45)
(45)
Sketch a phase diagram for water and correctly label all the parts (areas, lines, points, key temperatures).
(Essay)
4.8/5  (44)
(44)
For molecules, atoms, or ions with the same mass, which of the following forces typically is the strongest intermolecular interaction?
(Multiple Choice)
4.7/5  (43)
(43)
The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the highest boiling point? 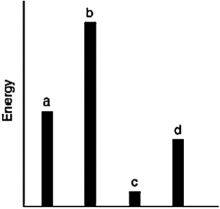
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following compounds will have the strongest dipole-dipole interactions between its molecules?
(Multiple Choice)
4.8/5  (34)
(34)
If the same liquid is present in each of the containers shown below, choose the solution with the highest rate of evaporation. Assume each container is full and has equal volume and only the top of the container is open. 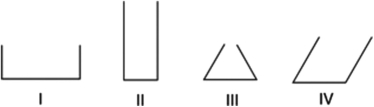
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following molecules has the highest boiling point?
(Multiple Choice)
4.8/5  (45)
(45)
Consider the phase diagram for a substance shown here. The line between the solid and liquid phases has a positive slope because the solid phase is ________ than the liquid phase. 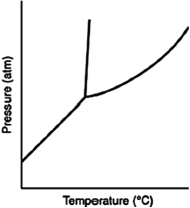
(Multiple Choice)
4.9/5  (34)
(34)
The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the lowest boiling point? 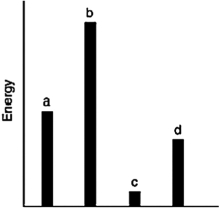
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following polar compounds is likely to have the highest boiling point?
(Multiple Choice)
5.0/5  (44)
(44)
Why does HI boil at a higher temperature than HBr, yet the dipole moment of HBr (0.82 D) is larger than that of HI (0.38 D)?
(Essay)
4.8/5  (33)
(33)
Which of the following compounds is capable of hydrogen bonding?
(Multiple Choice)
4.8/5  (39)
(39)
CH2F2 has a dipole moment of 1.93 D and a boiling point of 52C. CH2Cl2 has a dipole moment of 1.60 D and a boiling point of 40C. The boiling point of dichloromethane is higher than that of difluoromethane because of ________
(Multiple Choice)
4.8/5  (44)
(44)
Arrange the first four halogens in order of increasing boiling point.
(Short Answer)
4.9/5  (48)
(48)
Showing 21 - 40 of 165
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)