Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which best describes the intermolecular forces present in NH3?
(Multiple Choice)
4.9/5  (31)
(31)
The pressure inside a bottle of carbonated beverage decreases when it is cooled in a refrigerator. What is the main reason for this change?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following compounds will not possess dipole-dipole interactions between its molecules?
(Multiple Choice)
4.8/5  (35)
(35)
The relative energies (strengths) of the intermolecular forces present in each of four different pure gases are shown in the figure below. Which gas will show the smallest deviation from ideal gas behavior? 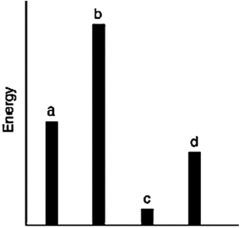
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following gases would be expected to most likely deviate from ideal gas behavior? Briefly explain your reasoning.
CH4, N2, and NH3
(Essay)
4.8/5  (46)
(46)
The Henry's law constant (mol/L · atm) for carbon dioxide dissolving in water is 3.50 102 mol/(L atm) at 20C. Calculate the molar concentration of carbon dioxide in a soda can where the air pressure is 2.45 atm. The mole fraction of carbon dioxide in air is 0.0397.
(Multiple Choice)
4.7/5  (35)
(35)
A solute is most likely to be highly soluble in a solvent if the solute is ________ and the solvent is ________.
(Multiple Choice)
4.8/5  (33)
(33)
Given the van der Waals a constant values for the following gases, which gas has the lowest boiling point?
(Multiple Choice)
4.8/5  (27)
(27)
Of all the noble gases, ________ has the strongest intermolecular force and hence the highest boiling point.
(Multiple Choice)
4.8/5  (39)
(39)
Which of the substances a-d in the following figure has the weakest intermolecular forces? 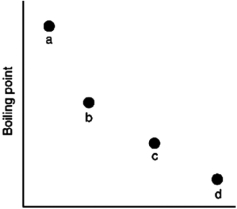
(Multiple Choice)
4.8/5  (30)
(30)
The density of water decreases as it is cooled from 4.0C to freezing because ________
(Multiple Choice)
4.8/5  (38)
(38)
Henry's law constant (mol/L · atm) for oxygen dissolving in blood is 3.74 102 mol/L · atm at body temperature, 37C. Calculate the molar concentration of oxygen in blood for an alpine climber where the atmospheric pressure is 0.45 atm. The mole fraction of oxygen in air is 0.209.
(Multiple Choice)
4.9/5  (40)
(40)
Of all the noble gases, ________ has the weakest intermolecular force and hence the lowest boiling point.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following diagrams best shows a set of polar molecules interacting through dipole-dipole interactions?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following representations best shows the formation of an instantaneous dipole moment between two nonpolar molecules?
(Multiple Choice)
4.7/5  (39)
(39)
A hydration sphere forms around an ion in aqueous solution due to ________
(Multiple Choice)
4.9/5  (40)
(40)
The solubility of any gas in a liquid can always be increased by ________
(Multiple Choice)
4.7/5  (31)
(31)
Showing 101 - 120 of 165
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)