Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance is most likely to be a solid at room temperature? 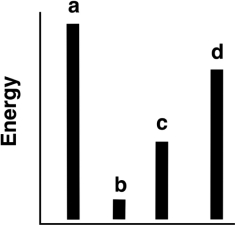
(Multiple Choice)
4.8/5  (26)
(26)
Dipole-dipole interactions typically are not as strong as ion-dipole interactions because ________
(Multiple Choice)
4.8/5  (40)
(40)
When potassium bromide dissolves in water, which picture best represents the solution? 
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following compounds would you most appropriately call hydrophobic?
(Multiple Choice)
4.9/5  (33)
(33)
Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the properties of isooctane (C8H18), which has an enthalpy of vaporization of 35.8 kJ/mol and a boiling point of 98.2C. Determine the vapor pressure of isooctane on a very hot summer day when the temperature is 38C.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following gases do you expect to be least soluble in a polar solvent?
(Multiple Choice)
4.8/5  (35)
(35)
For the following unsaturated phospholipid, identify the hydrophilic and hydrophobic regions of the molecule and explain the rationale for your choice. 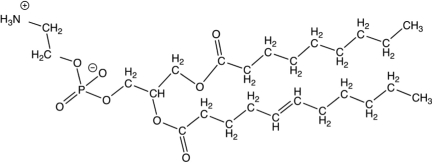
(Essay)
4.9/5  (43)
(43)
Which type of intermolecular interaction exists for all compounds?
(Multiple Choice)
5.0/5  (36)
(36)
For molecules or atoms with the same mass, which of the following typically is the weakest intermolecular interaction?
(Multiple Choice)
4.8/5  (38)
(38)
At the point marked with a dot on the phase diagram, the solid will ________ 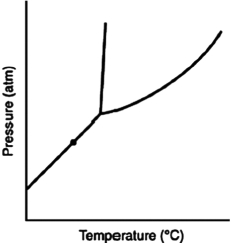
(Multiple Choice)
4.8/5  (33)
(33)
The boiling point of HBr is higher than that of HCl because HBr has ________ (The dipole moments are 1.08 D for HCl and 0.82 D for HBr.)
(Multiple Choice)
4.8/5  (35)
(35)
Which statement about vapor pressure of a pure liquid is not correct?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statements correctly characterizes the rate of evaporation of a liquid?
I. Increases with increasing temperature because molecules have higher kinetic energies.
II. Increases with increasing surface area of the liquid because more molecules are located at the surface.
III. Decreases with stronger intermolecular forces because molecules are less able to escape from the surface of the liquid.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following substances has a solid whose freezing point will decrease with increasing pressure?
(Multiple Choice)
4.7/5  (30)
(30)
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the lowest melting point? 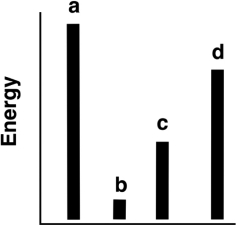
(Multiple Choice)
4.8/5  (31)
(31)
Identify the dominant intermolecular interaction(s) that must be overcome when solid CO2 sublimes and H2O melts.
(Essay)
4.9/5  (32)
(32)
Showing 61 - 80 of 165
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)