Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which arrangement is correct for increasing atomic radius?
(Multiple Choice)
4.8/5  (42)
(42)
Which arrangement is not the correct order of increasing first ionization energy?
(Multiple Choice)
4.8/5  (32)
(32)
De Broglie proposed that h/mv. Define the symbols in this equation and describe the significance of de Broglie's proposal.
(Essay)
4.9/5  (29)
(29)
What is the orbital designation for an electron with the quantum numbers n  4,
4, 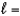 3?
3?
(Multiple Choice)
4.8/5  (31)
(31)
Write the electron configuration for each of the following atoms: a) Zn b) C c) P d) Sr.
(Essay)
4.8/5  (28)
(28)
Recently, buckyballs (C60) became the largest objects with a measured de Broglie wavelength. If the mass of a C60 molecule is 1.20 1024 kg, what will be its de Broglie wavelength if it is moving at a speed of 220 m/sec?
(Short Answer)
4.9/5  (40)
(40)
Which of the following electron configurations represents an excited state?
(Multiple Choice)
4.9/5  (40)
(40)
When the principle quantum number 3, what values are possible for the other quantum numbers 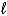 and m
and m 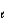 ?
?
(Essay)
4.9/5  (34)
(34)
What is the energy (E, in J) of a photon from a microwave oven with a frequency of 6.0 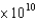 Hz?
Hz?
(Short Answer)
5.0/5  (43)
(43)
How many unpaired electrons does the nitride (N3) ion have?
(Multiple Choice)
4.9/5  (38)
(38)
A certain shell is known to have a total of 9 orbitals. Which shell is it?
(Multiple Choice)
4.9/5  (34)
(34)
What is the minimum frequency of a photon that can eject a photoelectron from Ba metal? (The work function of barium is 4.3 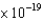 J.)
J.)
(Multiple Choice)
4.9/5  (29)
(29)
What is the ground-state electron configuration of a Cl ion?
(Multiple Choice)
4.7/5  (32)
(32)
Which statement about the quantum numbers that identify an atomic orbital is not correct?
(Multiple Choice)
4.8/5  (30)
(30)
What color will a blue object appear when it is seen through a filter with the absorption spectrum shown below? 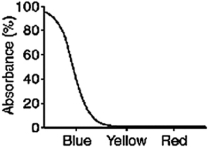
(Multiple Choice)
4.7/5  (43)
(43)
Showing 101 - 120 of 166
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)