Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which statement about the quantum numbers that identify an atomic orbital is not correct?
(Multiple Choice)
4.8/5  (39)
(39)
Which statement A-D about the wave function for a single electron is not correct?
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following electrons will have the smallest de Broglie wavelength?
(Multiple Choice)
4.8/5  (45)
(45)
Determine the wavelength of the line in the hydrogen atom spectrum corresponding to the n1 2 to n2 6 transition.
(Multiple Choice)
4.9/5  (43)
(43)
Recently, buckyballs (C60) became the largest objects with a measured de Broglie wavelength. If the mass of a C60 molecule is 1.20 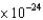 kg, what will be its de Broglie wavelength if it is moving at a speed of 220 m/sec?
kg, what will be its de Broglie wavelength if it is moving at a speed of 220 m/sec?
(Multiple Choice)
4.9/5  (36)
(36)
The studies of light emitted by hot objects (Max Planck) and the photoelectric effect (Albert Einstein) revolutionized physics because they revealed ________
(Multiple Choice)
4.9/5  (37)
(37)
A proton in a cyclotron has a velocity of 2 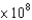 m/s, which is nearly the speed of light. The uncertainty in the velocity is 1%. What is the minimum uncertainty in the position of the proton in meters? The proton mass is 2
m/s, which is nearly the speed of light. The uncertainty in the velocity is 1%. What is the minimum uncertainty in the position of the proton in meters? The proton mass is 2 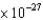 kg.
kg.
(Multiple Choice)
5.0/5  (44)
(44)
In comparing the dark Fraunhofer lines with light emitted by elements in a flame, Bunsen and Kirchhoff demonstrated that ________
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following orbitals is the highest in energy (assuming they are all in the same shell)?
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following elements has the ground-state electron configuration 1s22s22p63s23p2?
(Multiple Choice)
4.8/5  (36)
(36)
What is the ground state electron configuration for fluorine?
(Multiple Choice)
4.9/5  (38)
(38)
Indicate which of the following sources produces the lowest energy photons.
(Multiple Choice)
4.9/5  (48)
(48)
The emission spectra of Na and Na are different because ________
(Multiple Choice)
4.8/5  (39)
(39)
Identify which element, potassium or bromine, has the larger electron affinity and explain why.
(Essay)
4.7/5  (39)
(39)
Which of the following elements has the ground-state electron configuration 1s22s22p3?
(Multiple Choice)
4.7/5  (41)
(41)
Which arrangement is not in the correct order of decreasing first ionization energy?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 121 - 140 of 166
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)