Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
What wavelength of light is required to cause the ejection of photoelectrons with kinetic energies of 5.0 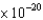 J from a calcium surface ( 4.60
J from a calcium surface ( 4.60 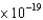 J)?
J)?
(Multiple Choice)
4.7/5  (37)
(37)
Based on the following graph, which metal will emit the highest energy photoelectron when a photon with a frequency of 3.9 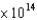 Hz is incident on the metal?
Hz is incident on the metal? 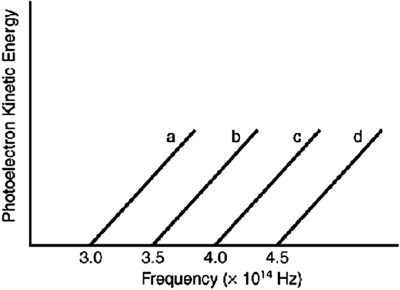
(Multiple Choice)
4.9/5  (37)
(37)
Down a column in the periodic table, the ionization energy ________
(Multiple Choice)
4.7/5  (31)
(31)
What is the energy (E, in J) of a photon from a microwave oven with a frequency of 6.0 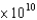 Hz?
Hz?
(Multiple Choice)
4.8/5  (31)
(31)
Define effective nuclear charge. Use this parameter to rationalize the general trends for atomic size down a group and across a period.
(Essay)
4.9/5  (47)
(47)
A light detector based on the photoelectric effect is made with cesium metal. Cesium metal has a work function of 3.40 1019 J. What is the longest wavelength of light that one could expect to detect with this device?
(Short Answer)
4.8/5  (41)
(41)
The mathematical description of an electron as a wave was developed by ________
(Multiple Choice)
4.9/5  (40)
(40)
An atom in its ground state absorbs a single photon of light and then relaxes back to the ground state by emitting an infrared photon (1,200 nm) followed by an orange photon (600 nm). What is the wavelength of the photon that was absorbed initially? 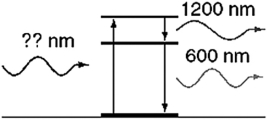
(Multiple Choice)
4.9/5  (40)
(40)
The electron configuration of a manganese ion (Z 25) is [Ar]3d 3. What is the charge on this ion?
(Multiple Choice)
4.8/5  (39)
(39)
How many orbitals that have the principal quantum number equal to 3 are there in an atom?
(Multiple Choice)
4.8/5  (31)
(31)
What is the wavelength of a photon emitted by a Kr laser with an energy of 3.07 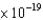 J?
J?
(Multiple Choice)
4.7/5  (34)
(34)
Provide three potential quantum numbers for the following orbitals.
a) 3d
b) 4s
c) 2p
(Essay)
4.9/5  (37)
(37)
Which of the following occurs only in discrete (quantized) increments?
(Multiple Choice)
4.8/5  (37)
(37)
The energy of a one-electron atom is given by 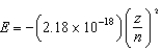 where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
(Multiple Choice)
4.8/5  (37)
(37)
The atomic radius of germanium (Z  32) is smaller than the atomic radius of potassium (Z
32) is smaller than the atomic radius of potassium (Z  19) because of ________
19) because of ________
(Multiple Choice)
4.9/5  (39)
(39)
What color will a red object appear when it is seen through a filter with the absorption spectrum shown below? 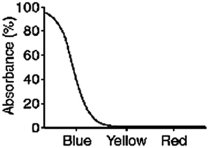
(Multiple Choice)
4.9/5  (29)
(29)
Showing 141 - 160 of 166
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)