Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which of the following objects, all moving at the same speed, will have the largest de Broglie wavelength?
(Multiple Choice)
4.7/5  (35)
(35)
Indicate which of the following photons can cause emission of photoelectrons from a surface of gallium ( 6.7 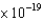 J).
J).
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following elements would you expect to have the lowest first ionization energy?
(Multiple Choice)
4.8/5  (41)
(41)
`If cesium, which has a work function of 2.1 eV, is used in a photodetector, what will be the kinetic energy of electrons produced by green light (540 nm) incident on the detector?
(1 eV  1.60
1.60 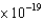 J)
J)
(Short Answer)
4.9/5  (41)
(41)
What is the kinetic energy of the photoelectrons emitted from a sodium surface ( 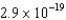 J) when it is irradiated by photons with a wavelength of 350 nm?
J) when it is irradiated by photons with a wavelength of 350 nm?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the transitions in the hydrogen atom energy-level diagram shown here is not possible? 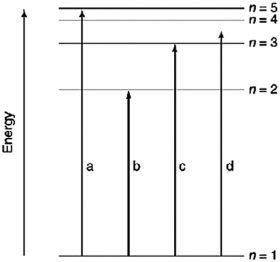
(Multiple Choice)
4.8/5  (36)
(36)
What is the minimum energy that a photon must have to ionize a hydrogen atom from the n 3 energy level?
(Short Answer)
4.8/5  (36)
(36)
What is the energy (E, in J) of the photons emitted by an Ar laser with a wavelength of 488 nm?
(Multiple Choice)
4.9/5  (35)
(35)
A certain shell is known to have a total of 16 orbitals. Which shell is it?
(Multiple Choice)
4.8/5  (36)
(36)
Across a row in the periodic table, the ionization energy ________
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following is the ground-state electron configuration of the Mg2 ion?
(Multiple Choice)
4.8/5  (36)
(36)
Which arrangement is in the correct order of decreasing radii?
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following diagrams represents the electron configuration of a carbon atom?
(Multiple Choice)
4.8/5  (34)
(34)
According to de Broglie, if the circumference of the electron's orbit in the hydrogen atom is twice the electron's wavelength, the orbit will ________
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following sources produces the lowest energy photons?
(Multiple Choice)
4.9/5  (35)
(35)
Arrange the following elements in order of increasing electron affinity: Li, O, Ne, Cl.
(Short Answer)
4.8/5  (41)
(41)
The study of light emitted by the hydrogen atom by Johann Balmer, Johannes Rydberg, Niels Bohr, and others revolutionized physics because it revealed that ________
(Multiple Choice)
4.9/5  (29)
(29)
Showing 41 - 60 of 166
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)