Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
At what temperature does the Fe(s) Fe(g) phase transition occur? H = 415.5 kJ/mol; S = 153.4 J/mol · K.
(Multiple Choice)
4.7/5  (41)
(41)
Heat transfer from the system to the surroundings has a large effect on Ssurr ___________
(Multiple Choice)
4.8/5  (39)
(39)
According to the second law of thermodynamics, the change in the entropy of the universe (Suniv) during a spontaneous reaction is __________
(Multiple Choice)
4.9/5  (32)
(32)
If 1 mol of ice melts at its melting point of 273 K, the entropy change for the ice is 22.0 J/K. If the ice melts in someone's hand at 34°C, what is the change in the entropy of the universe? Assume a final temperature for the water of 0°C. The enthalpy of fusion for ice is 6.01 kJ/mol.
(Multiple Choice)
4.9/5  (36)
(36)
The standard entropy of diamond is 2.4 J/mol · K. Calculate the entropy per carbon atom in diamond and the number of microstates for each atom. Discuss the number of microstates in terms of the molecular motions accessible to each carbon atom.
(Essay)
4.8/5  (32)
(32)
Give an example of a spontaneous and a nonspontaneous chemical process and the sign of the entropy change for the universe for each.
(Essay)
4.8/5  (36)
(36)
The equilibrium vapor pressure for benzene is 94.4 mm Hg. When liquid benzene is in equilibrium with its vapor, we must have __________
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following must be true for a spontaneous exothermic process?
(Multiple Choice)
4.9/5  (34)
(34)
Indicate which of the following has the lowest standard molar entropy (S°).
(Multiple Choice)
4.9/5  (38)
(38)
The entropy change in a system (Ssys) during a spontaneous process must be __________
(Multiple Choice)
4.8/5  (39)
(39)
Indicate which of the following has the highest entropy at 298 K.
(Multiple Choice)
4.8/5  (37)
(37)
What is the entropy change to the surroundings when 1 mol of ice melts in someone's hand if the hand temperature is 32°C? Assume a final temperature for the water of 0°C. The heat of fusion of ice is 6.01 kJ/mol.
(Multiple Choice)
4.7/5  (34)
(34)
Considering the tabulated values for the thermodynamic properties of oxygen gas, why is the standard entropy nonzero but the standard enthalpy and free energy of formation are zero?
(Essay)
4.8/5  (37)
(37)
The standard molar entropy of lead(II) bromide (PbBr2) is 161 J/mol · K. What is the entropy of 2.45 g of PbBr2?
(Multiple Choice)
4.8/5  (38)
(38)
Determine Grxn for C4H10( 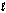 ) +
) + 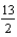 O2(g) 4CO2(g) + 5H2O(g) given the following. Substance
G
O2(g) 4CO2(g) + 5H2O(g) given the following. Substance
G  (J/mol · K)
C4H10(
(J/mol · K)
C4H10( 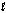 )
-15)0
CO2(g)
-394.4
H2O(g)
-228.57
)
-15)0
CO2(g)
-394.4
H2O(g)
-228.57
(Multiple Choice)
4.9/5  (36)
(36)
The standard molar enthalpy of fusion for xenon is 2.30 kJ mol-1. Calculate the standard molar entropy for the freezing of liquid xenon given that its normal freezing point is -112°C.
(Short Answer)
4.8/5  (43)
(43)
Benzene is a liquid under standard conditions. The standard free-energy change for the evaporation of liquid benzene to form benzene vapor is +5.2 kJ/mol. If liquid benzene is placed in an open container under standard conditions, __________
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following is in the correct order of standard state entropy?
(I)Diamond < graphite
(II)Liquid water < solid water
(III)NH3 < H2
(Multiple Choice)
4.8/5  (39)
(39)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)