Exam 42: Nuclear Physics
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion31 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Impulse and Momentum20 Questions
Exam 10: Energy43 Questions
Exam 11: Work100 Questions
Exam 12: Rotation of a Rigid Body113 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Oscillations49 Questions
Exam 15: Fluids and Elasticity72 Questions
Exam 16: A Macroscopic Description of Matter29 Questions
Exam 17: Work, Heat, and the First Law of Thermodynamics98 Questions
Exam 18: The Micromacro Connection39 Questions
Exam 19: Heat Engines and Refrigerators50 Questions
Exam 20: Traveling Waves49 Questions
Exam 21: Superpositions64 Questions
Exam 22: Wave Optics51 Questions
Exam 23: Ray Optics63 Questions
Exam 24: Optical Instruments49 Questions
Exam 25: Electric Charges and Forces26 Questions
Exam 26: The Electric Field32 Questions
Exam 27: Gausss Law41 Questions
Exam 28: The Electric Potential40 Questions
Exam 29: Potential and Field57 Questions
Exam 30: Current and Resistance32 Questions
Exam 31: Fundamentals of Circuits68 Questions
Exam 32: The Magnetic Field87 Questions
Exam 33: Electromagnetic Induction66 Questions
Exam 34: Electromagnetic Fields and Waves52 Questions
Exam 35: Ac Circuits46 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics8 Questions
Exam 38: Quantization54 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics39 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
Carbon-14 has a half-life of 5730 y. A sample of wood has been recovered by an archaeologist. The sample is sent to a laboratory, where it is determined that the activity of the sample is 0.144 Bq/g. By comparing this activity with the activity of living organic matter, 0.230 Bq/g, the scientist determines how old the wood sample is, or more precisely, when the tree that the sample came from died. How old is the sample of wood?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
A
What is the binding energy per nucleon for 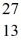 Al? The neutral
Al? The neutral 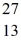 Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. (1 u = 931.494 MeV/c2)
Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. (1 u = 931.494 MeV/c2)
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
A
In a laboratory accident a work area is contaminated with radioactive material. Health physicists monitor the area during a 30-day period and, after correcting for the background rate, obtain the data shown in the table.  The accident occurred at t = 0. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute. Of the choices listed below, which one is the earliest time that workers could safely return?
The accident occurred at t = 0. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute. Of the choices listed below, which one is the earliest time that workers could safely return?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
A
If a nucleus had a diameter of 8.0 fm, what would be its expected mass, in atomic mass units?
(Multiple Choice)
4.9/5  (32)
(32)
A radioisotope has a half-life of τ at a temperature of 150 K. If its temperature is increased to 300 K, what will its half-life be?
(Multiple Choice)
4.8/5  (39)
(39)
A certain isotope has a half-life of 32.4 hr and a relative biological effectiveness of 3.50. A sample of this isotope initially delivers an absorbed dose of 0.240 Gy to 250 g of tissue.
(a) What was the initial equivalent dose to the tissue in rem and in sieverts?
(b) How many joules of energy did the 250-g sample initially receive from the isotope?
(Short Answer)
4.8/5  (22)
(22)
Which of the following statements about the strong nuclear force are correct? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (32)
(32)
A certain nucleus containing 8 protons and 7 neutrons has a radius R. Which of the following values would be closest to the expected value of the radius of a nucleus having 51 protons and 69 neutrons?
(Multiple Choice)
4.8/5  (48)
(48)
The radioactive nuclei 60Co is widely used in medical applications. It undergoes beta decay, and the total energy of the decay process is 2.82 MeV per decay event. The half-life of this nucleus is 272 days. Suppose that a patient is given a dose of 6.9 µCi of 60Co. If all of this material decayed while in the patient's body, what would be the total energy deposited there? (1 Ci = 3.70 × 1010 decays/s)
(Multiple Choice)
4.8/5  (30)
(30)
What would be the expected radius of a nucleus having 82 protons and 125 neutrons?
(Multiple Choice)
4.7/5  (34)
(34)
How much energy is released when 1.40 μg of 3H have decayed to 3He? The mass of 3He is 3.016029 u, the mass of 3H is 3.016049 u, and 1 u = 931.494 MeV/c2.
(Multiple Choice)
4.8/5  (33)
(33)
A hospital patient has been given some 131I (half-life = 8.04 d) which decays at 4.2 times the acceptable level for exposure to the general public. How long must the patient wait for the decay rate to reach the acceptable level? Assume that the material merely decays and is not excreted by the body.
(Multiple Choice)
5.0/5  (38)
(38)
Living matter has 1.3 × 10-10 % of its carbon in the form of 14C which has a half-life of 5730 y. A mammoth bone has a 300-g sample of carbon separated from it, and the sample is found to have an activity of 20 decays per second. How old is the bone?
(Multiple Choice)
4.9/5  (32)
(32)
A stationary plutonium-239 nucleus decays into a uranium-235 nucleus plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values 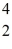 He: 4.002603 u
He: 4.002603 u 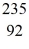 U: 235.043924 u
What is the kinetic energy of the
U: 235.043924 u
What is the kinetic energy of the 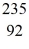 U nucleus? (1 u =931.494 MeV/c2)
U nucleus? (1 u =931.494 MeV/c2)
(Multiple Choice)
4.8/5  (24)
(24)
The carbon in your body was formed in nuclear reactions in long-dead stars. How much energy was released when three 4He nuclei combined to make 12C? The mass of 4He is 4.002603 u, the mass of 12C is 12.0000 u, and 1 u = 931.494 MeV/c2.
(Multiple Choice)
4.9/5  (43)
(43)
A radioactive sample has a half-life of 10 min. What fraction of the sample is left after 40 min?
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following statements about the atomic nucleus is correct? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (42)
(42)
The following masses are known: 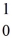 n (neutron) 1.008665 u
n (neutron) 1.008665 u 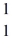 H 1.007825 u
H 1.007825 u 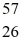 Fe 56.935399 u
What is the binding energy of
Fe 56.935399 u
What is the binding energy of 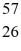 Fe, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Fe, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
(Multiple Choice)
4.8/5  (33)
(33)
Modern nuclear bomb tests have created an extra high level of 14C in our atmosphere. Suppose that future archaeologists date samples from our era, but do not know about this testing. Will their dates be too young, too old, or still correct? If correct they are correct, why?
(Multiple Choice)
4.9/5  (37)
(37)
A sphere made of a radioactive isotope initially has a mass of 6.88 kg. The half-life of this isotope is 1.34 h, and it decays by β- emission. At the end of 2.68 h, what is the mass of this sphere?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 1 - 20 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)