Exam 18: The Micromacro Connection
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion31 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Impulse and Momentum20 Questions
Exam 10: Energy43 Questions
Exam 11: Work100 Questions
Exam 12: Rotation of a Rigid Body113 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Oscillations49 Questions
Exam 15: Fluids and Elasticity72 Questions
Exam 16: A Macroscopic Description of Matter29 Questions
Exam 17: Work, Heat, and the First Law of Thermodynamics98 Questions
Exam 18: The Micromacro Connection39 Questions
Exam 19: Heat Engines and Refrigerators50 Questions
Exam 20: Traveling Waves49 Questions
Exam 21: Superpositions64 Questions
Exam 22: Wave Optics51 Questions
Exam 23: Ray Optics63 Questions
Exam 24: Optical Instruments49 Questions
Exam 25: Electric Charges and Forces26 Questions
Exam 26: The Electric Field32 Questions
Exam 27: Gausss Law41 Questions
Exam 28: The Electric Potential40 Questions
Exam 29: Potential and Field57 Questions
Exam 30: Current and Resistance32 Questions
Exam 31: Fundamentals of Circuits68 Questions
Exam 32: The Magnetic Field87 Questions
Exam 33: Electromagnetic Induction66 Questions
Exam 34: Electromagnetic Fields and Waves52 Questions
Exam 35: Ac Circuits46 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics8 Questions
Exam 38: Quantization54 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics39 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
Eleven molecules have speeds 16, 17, 18, . . . , 26 m/s. Calculate the root-mean-square of this group of molecules.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
A
A 5.0-liter gas tank holds 1.4 moles of helium (He) and 0.70 moles of oxygen (O2), at a temperature of 260 K. The atomic masses of helium and oxygen are 4.0 g/mol and 16.0 g/mol, respectively. Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K. The total random translational kinetic energy of the gas in the tank is closest to
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
The root-mean-square speed (thermal speed) of the molecules of a gas is 200 m/s at 23.0°C. At 227°C the root-mean-square speed (thermal speed) of the molecules will be closest to
Free
(Multiple Choice)
4.7/5  (20)
(20)
Correct Answer:
C
A container is filled with a mixture of helium (light molecules) and oxygen (heavy molecules) gases. A thermometer in the container reads 22°C. Which gas molecules have the greater average kinetic energy?
(Multiple Choice)
5.0/5  (39)
(39)
As a result of any natural process, the total entropy of any system plus that of its environment
(Multiple Choice)
4.8/5  (36)
(36)
According to the second law of thermodynamics, the entropy of any system always increases.
(True/False)
4.8/5  (30)
(30)
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed) of 200 m/s. The mass of a helium molecule is 3.40 × 10-27 kg. What is the average pressure exerted by the molecules on the walls of the container? The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K.
(Multiple Choice)
4.8/5  (37)
(37)
The root-mean-square speed (thermal speed) for a certain gas at 100°C is 0.500 km/s. If the temperature of the gas is now increased to 200°C, the root-mean-square (thermal) speed will be closest to
(Multiple Choice)
4.9/5  (39)
(39)
An ideal gas is kept in a rigid container. When its temperature is 100 K, the mean-free-path of the gas molecules is λ. What will be the mean-free-path of the molecules at 400 K?
(Multiple Choice)
4.8/5  (38)
(38)
An ideal gas is kept in a rigid container that expands negligibly when heated. The gas starts at a temperature of 20.0°C, and heat is added to increase its temperature. At what temperature will its root-mean-square speed (thermal speed) be double its value at 20.0°C?
(Multiple Choice)
4.9/5  (32)
(32)
A mole of oxygen (O2) molecules and a mole of carbon dioxide (CO2) molecules at the same temperature and pressure have
(Multiple Choice)
4.7/5  (33)
(33)
A 0.10- 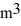 gas tank holds 5.0 moles of nitrogen gas (N2), at a temperature of 370 K. The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
gas tank holds 5.0 moles of nitrogen gas (N2), at a temperature of 370 K. The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
(Multiple Choice)
4.7/5  (39)
(39)
Dust particles are pulverized rock, which has density 2500 kg/m3. They are approximately spheres 20 μm in diameter. Treating dust as an ideal gas, what is the root-mean-square speed (thermal speed) of a dust particle at 400°C? (The Boltzmann constant is 1.38 × 10-23 J/K.)
(Multiple Choice)
4.9/5  (32)
(32)
Assuming the radius of diatomic molecules is approximately 1.0 × 10-10 m, for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K.
(Multiple Choice)
5.0/5  (33)
(33)
The root-mean-square speed (thermal speed) of the molecules of a gas is 200 m/s at a temperature 23.0°C. What is the mass of the individual molecules? The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.7/5  (39)
(39)
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in pressure. If the original root-mean-square speed (thermal speed) of the gas molecules was V, the new speed is
(Multiple Choice)
4.8/5  (35)
(35)
The mean free path of an oxygen molecule is 2.0 × 10-5 m, when the gas is at a pressure of 120 Pa and a temperature of 275 K. The molecular mass of oxygen is 32.0 g/mol and the Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K. Assuming that the molecules are moving at their root-mean-square speeds, the average time interval between collisions of an oxygen molecule is closest to
(Multiple Choice)
4.9/5  (43)
(43)
What is the mean free path of molecules in an ideal gas in which the mean collision time is 3.00 × 10-10 s, the temperature is 300 K, and the mass of the molecules is 6.00 × 10-25 kg? Assume that the molecules are moving at their root-mean-square speeds. The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.9/5  (29)
(29)
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature. What happens to the average speed of the molecules in the sample?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 1 - 20 of 39
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)