Exam 7: Quantum Theory and Atomic Structure
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
According to the Bohr theory of the hydrogen atom, the minimum energy (in J) needed to ionize a hydrogen atom from the n = 2 state is
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
Which of the following frequencies of electromagnetic radiation has the shortest wavelength?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
B
In the Bohr model of the hydrogen atom, the electron moves in a circular path which Bohr referred to as an orbital.
Free
(True/False)
4.9/5  (39)
(39)
Correct Answer:
False
Which of the following is a correct set of quantum numbers for an electron in a 3d orbital?
(Multiple Choice)
4.9/5  (29)
(29)
A photon has an energy of 5.53 × 10-17 J. What is its frequency in s-1?
(Multiple Choice)
4.7/5  (41)
(41)
A sprinter must average 24.0 mi/h to win a 100-m dash in 9.30 s. What is his wavelength at this speed if his mass is 84.5 kg?
(Multiple Choice)
4.8/5  (36)
(36)
Use the Rydberg equation to calculate the frequency of a photon absorbed when the hydrogen atom undergoes a transition from n1 = 2 to n2 = 4. (R = 1.096776 × 107 m-1)
(Multiple Choice)
4.8/5  (36)
(36)
Which scientist first proposed that the electron in the hydrogen atom can have only certain energies?
(Multiple Choice)
4.8/5  (36)
(36)
Green light has a wavelength of 5200 Å. Calculate the energy of one photon of green light.
(Multiple Choice)
4.8/5  (34)
(34)
The de Broglie equation predicts that the wavelength (in m) of a proton moving at 1000. m/s is
(Multiple Choice)
4.9/5  (28)
(28)
Infrared radiation from the sun has a wavelength of 6200 nm. Calculate the energy of one photon of that radiation.
(Multiple Choice)
4.8/5  (36)
(36)
Platinum, which is widely used as a catalyst, has a work function 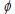 (the minimum energy needed to eject an electron from the metal surface) of 9.05 × 10-19 J. What is the longest wavelength of light which will cause electrons to be emitted?
(the minimum energy needed to eject an electron from the metal surface) of 9.05 × 10-19 J. What is the longest wavelength of light which will cause electrons to be emitted?
(Multiple Choice)
4.8/5  (35)
(35)
The AM station KBOR plays your favorite music from the 20's and 30's at 1290 kHz. Find the wavelength of these waves.
(Multiple Choice)
4.9/5  (34)
(34)
The energy of a photon is directly proportional to the wavelength of the radiation.
(True/False)
4.9/5  (37)
(37)
Line spectra from all regions of the electromagnetic spectrum, including the Paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. Calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (R = 1.096776 × 107 m-1)
(Multiple Choice)
4.8/5  (39)
(39)
The orientation in space of an atomic orbital is associated with
(Multiple Choice)
4.9/5  (32)
(32)
Showing 1 - 20 of 69
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)