Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain. Calculate the mass in grams of 3.65 × 1020 molecules of SO3.
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
C
Alkanes are compounds of carbon and hydrogen with the general formula CnH2n+2. An alkane component of gasoline has a molar mass of between 125 and 130 g/mol. What is the value of n for this alkane?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
How many atoms are in a drop of mercury that has a diameter of 1.0 mm? (Volume of a sphere is 4πr3/3; density of mercury = 13.6 g/cm3)
Free
(Multiple Choice)
5.0/5  (34)
(34)
Correct Answer:
A
Aluminum sulfate, Al2(SO4)3, is used in tanning leather, purifying water, and manufacture of antiperspirants. Calculate its molar mass.
(Multiple Choice)
4.9/5  (34)
(34)
Constitutional (structural) isomers have the same empirical formula but different molecular formulas.
(True/False)
4.7/5  (39)
(39)
Hydroxylamine nitrate contains 29.17 mass % N, 4.20 mass % H, and 66.63 mass O. If its molar mass is between 94 and 98 g/mol, what is its molecular formula?
(Multiple Choice)
4.8/5  (30)
(30)
What is the mass in grams of 0.250 mol of the common antacid calcium carbonate?
(Multiple Choice)
4.8/5  (30)
(30)
Terephthalic acid, used in the production of polyester fibers and films, is composed of carbon, hydrogen, and oxygen. When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O. What is its empirical formula?
(Multiple Choice)
4.8/5  (31)
(31)
In combustion analysis, the oxygen content of a substance is equal to the total oxygen in the CO2 and H2O collected in the absorbers.
(True/False)
4.8/5  (38)
(38)
Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3Mg(s) + 2FeCl3(s) → 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 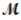 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 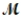 = 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
= 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
(Multiple Choice)
4.8/5  (39)
(39)
Terephthalic acid, used in the production of polyester fibers and films, is composed of carbon, hydrogen, and oxygen. When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O. If its molar mass is between 158 and 167 g/mol, what is its molecular formula?
(Multiple Choice)
4.8/5  (33)
(33)
Methanol (CH4O) is converted to bromomethane (CH3Br) as follows: CH4O + HBr → CH3Br + H2O
If 12.23 g of bromomethane are produced when 5.00 g of methanol is reacted with excess HBr, what is the percentage yield?
(Multiple Choice)
4.7/5  (36)
(36)
In combustion analysis, the carbon and hydrogen contents of a substance are determined from the CO2 and H2O, respectively, which are collected in the absorbers.
(True/False)
4.8/5  (43)
(43)
Aluminum will react with bromine to form aluminum bromide (used as an acid catalyst in organic synthesis). Al(s) + Br2(l) → Al2Br6(s) [unbalanced]
How many moles of Al are needed to form 2.43 mol of Al2Br6?
(Multiple Choice)
4.9/5  (30)
(30)
Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium chloride and iron. 3Mg(s) + 2FeCl3(s) → 3MgCl2(s) + 2Fe(s) A mixture of 41.0 g of magnesium ( 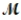 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 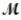 = 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
(Multiple Choice)
4.7/5  (39)
(39)
A compound containing chromium and silicon contains 73.52 mass percent chromium. Determine its empirical formula.
(Multiple Choice)
4.8/5  (28)
(28)
Balance the following equation: Ca3(PO4)2(s) + SiO2(s) + C(s) → CaSiO3(s) + CO(g) + P4(s)
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)