Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Which of the following should have the greatest molar entropy at 298 K?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
D
The reaction of methane with water to form carbon dioxide and hydrogen is nonspontaneous at 298 K. At what temperature will this system make the transition from nonspontaneous to spontaneous? The data refer to 298 K. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g)
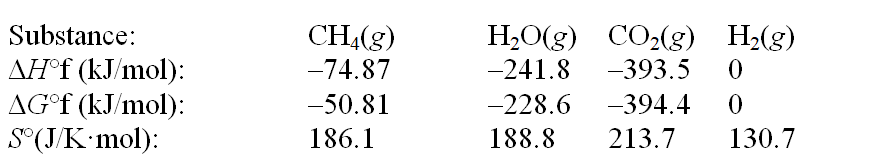
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
Which relationship or statement best describes ΔS° for the following reaction? 2NH3(g) + 2ClF3(g) → 6HF(g) + N2(g) + Cl2(g)
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
C
Which relationship or statement best describes ΔS° for the following reaction? CaO(s) + CO2(g) → CaCO3(s)
(Multiple Choice)
4.8/5  (31)
(31)
A reaction has ΔG = 10.0 kJ and ΔG° = 15.0 kJ at a temperature of 50°C. Calculate the value of the reaction quotient Q under these conditions.
(Multiple Choice)
4.9/5  (38)
(38)
Elemental boron can be formed by reaction of boron trichloride with hydrogen. BCl3(g) + 1.5H2(g) → B(s) + 3HCl(g)
Substance: BCl3(g) H2(g) B(s) HCl(g)
S°(J/K·mol): ? 130.6 5.87 186.8
If ΔS° = 80.3 J/K for the reaction above, what is S° for BCl3(g)?
(Multiple Choice)
4.9/5  (35)
(35)
Given: H2O(l) → H2O(s) ΔH° = -6.02 kJ at 273K Calculate the entropy change of the surroundings (ΔSsurr) when one mole of water freezes at 0°C and a pressure of one atmosphere.
(Multiple Choice)
4.8/5  (32)
(32)
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. H3PO4(s)  H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.
H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.
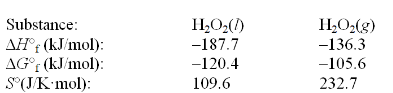
(Multiple Choice)
4.9/5  (45)
(45)
Which relationship or statement best describes ΔS° for the following reaction? Pb(s) + Cl2(g) → PbCl2(s)
(Multiple Choice)
4.8/5  (48)
(48)
Which relationship or statement best describes ΔS° for the following reaction? BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
(Multiple Choice)
4.8/5  (36)
(36)
Calculate ΔG° for the combustion of propane. C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
Substance: C3H8(g) O2(g) CO2(g) H2O(g)
ΔG°f (kJ/mol): -24.5 0 -394.4 -228.6
(Multiple Choice)
5.0/5  (40)
(40)
Under a given set of conditions, all microstates of a system are equally probable.
(True/False)
4.8/5  (34)
(34)
For a chemical reaction to be spontaneous only at high temperatures, which of the following conditions must be met?
(Multiple Choice)
4.7/5  (39)
(39)
Iron(III) oxide can be reduced by carbon monoxide. Fe2O3(s) + 3CO(g)  2Fe(s) + 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature.
2Fe(s) + 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature.
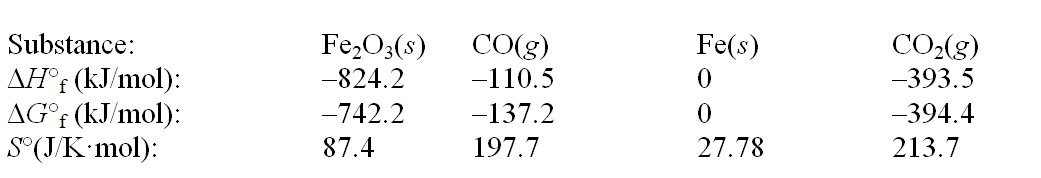
(Multiple Choice)
4.9/5  (37)
(37)
As a chemical reaction proceeds toward equilibrium, the free energy of the system decreases.
(True/False)
4.9/5  (37)
(37)
Which relationship or statement best describes ΔS° for the following reaction? C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
(Multiple Choice)
4.9/5  (30)
(30)
Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine. The data refer to 298 K. SO2(g) + Cl2(g) → SO2Cl2(g)
Substance: SO2(g) Cl2(g) SO2Cl2(g)
ΔH°f (kJ/mol): -296.8 0 -364.0
ΔG°f (kJ/mol): -300.1 0 -320.0
S°(J/K·mol): 248.2 223.0 311.9
What is the value of ΔG° for this reaction at 600 K?
(Multiple Choice)
4.9/5  (35)
(35)
You are given pure samples of ammonia, NH3(g), and nitrogen trifluoride, NF3(g). What prediction would you make concerning their standard molar entropies at 298 K?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)