Exam 13: The Properties of Mixtures Solutions and Colloids
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
If the density of a solution is less than 1.0 g/mL, its molarity will be greater than its molality.
Free
(True/False)
4.8/5  (30)
(30)
Correct Answer:
False
Procaine hydrochloride (  = 272.77 g/mol) is used as a local anesthetic. Calculate the molarity of a 4.666 m solution which has a density of 1.1066 g/mL.
= 272.77 g/mol) is used as a local anesthetic. Calculate the molarity of a 4.666 m solution which has a density of 1.1066 g/mL.
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
A
Two aqueous are prepared: 1.00 m Na2CO3 and 1.00 m LiCl. Which of the following statements is true?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
B
What concentration of aqueous FeCl3 would have the same osmotic pressure as a 0.20 M solution of CaCl2 at the same temperature, assuming ideal behavior?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following statements describes the correct method of preparation of 1.00 L of a 2.0 M urea solution? 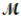 urea = 60.06 g/mol
urea = 60.06 g/mol
(Multiple Choice)
4.7/5  (36)
(36)
What is the mole fraction of Ne in a mixture containing 10.1 g of Ne, 79.9 g of Ar, and 83.8 g of Kr?
(Multiple Choice)
5.0/5  (40)
(40)
Electrolyte solutions generally behave less ideally as the solute concentration increases.
(True/False)
4.7/5  (42)
(42)
How many moles of sulfate ions are present in 1.0 L of 0.5 M Li2SO4?
(Multiple Choice)
4.8/5  (34)
(34)
Dimethylglyoxime, DMG, is an organic compound used to test for aqueous nickel(II) ions. A solution prepared by dissolving 65.0 g of DMG in 375 g of ethanol boils at 80.3°C. What is the molar mass of DMG? Kb = 1.22°C/m, boiling point of pure ethanol = 78.5°C
(Multiple Choice)
4.8/5  (29)
(29)
A saturated solution of carbon dioxide in water contains 3.00 g of CO2 when the CO2 partial pressure is 8.0 atm. What mass of CO2 will escape if the partial pressure is lowered to 3.2 atm?
(Multiple Choice)
4.8/5  (30)
(30)
The vapor pressure of pure acetone (propanone) is 266 torr. When a non-volatile solute is added, the vapor pressure of acetone above the solution falls to 232 torr. What is the mole fraction of acetone in the solution?
(Multiple Choice)
4.9/5  (35)
(35)
Which, if any, of the following features is common to soaps, detergents, phospholipids, and channel-forming antibiotics?
(Multiple Choice)
4.8/5  (42)
(42)
A 2.0% (w/v) solution of sodium hydrogen citrate, Na2C6H6O7, which also contains 2.5% (w/v) of dextrose, C6H12O6, is used as an anticoagulant for blood which is to be used for transfusions. What is the molarity of the sodium hydrogen citrate in the solution?
(Multiple Choice)
5.0/5  (31)
(31)
For a given solution, which of the following concentration values will change as temperature changes?
(Multiple Choice)
4.9/5  (33)
(33)
Which one of the following pairs of dispersed phases and dispersing media can never form a colloid?
(Multiple Choice)
4.8/5  (33)
(33)
The shape of a protein molecule is determined completely by
(Multiple Choice)
4.7/5  (30)
(30)
Which of the following ions will be expected to have the most negative heat of hydration, ΔHhydr?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following sets of conditions could exist when two liquids which are completely miscible in one another are mixed?
(Multiple Choice)
4.7/5  (34)
(34)
Showing 1 - 20 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)