Exam 5: Gases and the Kinetic-Molecular Theory
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Hydrochloric acid is prepared by bubbling hydrogen chloride gas through water. What is the concentration of a solution prepared by dissolving 225 L of HCl(g) at 37°C and 89.6 kPa in 5.25 L of water?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
A
Which of the following gases effuses most rapidly?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
D
A 0.850-mole sample of nitrous oxide, a gas used as an anesthetic by dentists, has a volume of 20.46 L at 123°C and 1.35 atm. What would be its volume at 468°C and 1.35 atm?
Free
(Multiple Choice)
5.0/5  (32)
(32)
Correct Answer:
D
A 500-mL sample of argon at 800 torr has its absolute temperature quadrupled. If the volume remains unchanged what is the new pressure?
(Multiple Choice)
4.9/5  (43)
(43)
According to the kinetic theory of gases, in a collision between two molecules the kinetic energy of one molecule will decrease by the same amount that the kinetic energy of the other one increases.
(True/False)
4.9/5  (42)
(42)
Calculate the temperature of an argon sample at 55.4 kPa and 18.6 L if it occupies 25.8 L at 75.0°C and 41.1 kPa.
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the root-mean-square speed of methane, CH4 (g), at 78°C.
(Multiple Choice)
4.9/5  (44)
(44)
Hydrogen peroxide was catalytically decomposed and 75.3 mL of oxygen gas was collected overwater at 25°C and 742 torr. What mass of oxygen was collected? (Pwater = 24 torr at 25°C)
(Multiple Choice)
4.8/5  (27)
(27)
A sample of nitrogen gas is confined to a 14.0 L container at 375 torr and 37.0°C. How many moles of nitrogen are in the container?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its pressure, other factors remaining constant? 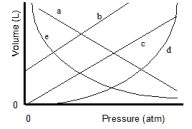
(Multiple Choice)
4.7/5  (30)
(30)
If the molecular mass of a gas increases by a factor of 4 at constant temperature, its rms speed will
(Multiple Choice)
4.9/5  (44)
(44)
A flask containing argon gas is connected to a closed-ended mercury manometer. The closed end is under vacuum. If the mercury level in the closed arm is 230. mm above that in the arm connected to the flask, what is the argon pressure, in torr?
(Multiple Choice)
4.9/5  (32)
(32)
A sample of carbon dioxide gas at 125°C and 248 torr occupies a volume of 275 L. What will the gas pressure be if the volume is increased to 321 L at 125°C?
(Multiple Choice)
4.8/5  (36)
(36)
If 0.750 L of argon at 1.50 atm and 177°C and 0.235 L of sulfur dioxide at 95.0 kPa and 63.0°C are added to a 1.00-L flask and the flask's temperature is adjusted to 25.0°C, what is the resulting pressure in the flask?
(Multiple Choice)
4.9/5  (43)
(43)
Mercury is 13.6 times as dense as liquid water. What would be the reading of a water-filled barometer at normal atmospheric pressure, 760. mmHg?
(Multiple Choice)
4.9/5  (36)
(36)
The air pressure in a volleyball is 75 psi. What is this pressure in torr?
(Multiple Choice)
4.8/5  (40)
(40)
A carbon dioxide sample weighing 44.0 g occupies 32.68 L at 65°C and 645 torr. What is its volume at STP?
(Multiple Choice)
4.9/5  (34)
(34)
A 3.0-L sample of helium was placed in a container fitted with a porous membrane. Half of the helium effused through the membrane in 24 hours. A 3.0-L sample of oxygen was placed in an identical container. How many hours will it take for half of the oxygen to effuse through the membrane?
(Multiple Choice)
4.9/5  (36)
(36)
"The total pressure in a mixture of unreacting gases is equal to the sum of the partial pressures of the individual gases" is a statement of __________________ Law.
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)