Exam 7: Quantum Theory and Atomic Structure
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
What type of spectrum, if any, would be produced if the light radiated by a heated atomic gas were to be dispersed through a prism?
(Multiple Choice)
4.8/5  (33)
(33)
A modern compact fluorescent lamp contains 1.4 mg of mercury. If each mercury atom in the lamp were to emit a single photon of wavelength 254 nm, how many joules of energy would be emitted?
(Multiple Choice)
4.9/5  (39)
(39)
Which one of the following sets of quantum numbers can correctly represent a 3p orbital?
(Multiple Choice)
4.9/5  (31)
(31)
Electromagnetic radiation can be specified by its wavelength ( ), its frequency ( ) or its period (). The period is the time it takes one complete wavelength to pass a point in space. Based on this information, what is the mathematical relationship between and ?
(Multiple Choice)
4.8/5  (25)
(25)
For all the allowed vibration(s) (wavelength(s)) of a plucked guitar string, what is the correct relationship between the length of the string, L, and the wavelength, 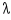 ?
?
(Multiple Choice)
5.0/5  (38)
(38)
If the energy of a photon is 1.32 × 10-18 J, what is its wavelength in nm?
(Multiple Choice)
4.9/5  (32)
(32)
Continuous spectra are characteristic of molecules in the gas phase.
(True/False)
4.8/5  (38)
(38)
In the Rydberg equation, for a fixed value of n1, the longest wavelength line has n2 =∞.
(True/False)
4.8/5  (34)
(34)
Showing 61 - 69 of 69
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)